Introduction
Materials and Methods
Materials
Selection of experimental animals and breeding environment
Cultivation conditions for strains and manufacturing of liquid spawn
Cultivation of mushroom mycelium in Aloe as a natural medium
pH value changes of the medium culturing intestinal bacteria containing aloe-fermented products
Inhibition activity against β-glucuronidase
Inhibition activity against Tryptophanase
The effects on improving inflammatory bowel disease induced by TNBS (Trinitrobenzene Sulfonic Acid)
Setting the injection volume and organization of the experimental groups
Observation and test items
Statistical methods
Results and Discussion
Changes of pH value in the medium for culturing intestinal bacteria containing aloe-fermented products
Inhibition effects against β-glucuronidase and tryptophanase
Improvement effects on inflammatory bowel disease (IBD) induced by trinitrobenzen sulfonic acid (TNBS) in a mouse model
Summary
Introduction
Inflammatory bowel disease (IBD) is repetitive and one of the most difficult gastrointestinal diseases to cure. Despite many studies from all over the world, causes of the disease, its pathological basis, and effective treatments have not been found. IBD is commonly clinically diagnosed with stomach ache, diarrhea, and pyemia liquid stools. Lesions appear on the mucosa and mucus membranes. Pathologically, ulcers are formed. Abnormal symptoms such as promontory abscesses, small blood vessel inflammation, goblet cell reduction, and various types of inflammatory cells appear. In particular, after ulcer formation, 13% of IBD patients are led to colon cancer. Causes of the disease are considered to be environmental, such as smoking and food, hereditary, and by bacterial infection. Each element can be involved independently or combined. Particularly in people where it’s hereditary, when genetically caused or by exposure to one of these elements, inflammation of the colon mucosa and immunity reaction incurs. When this reaction does not disappear and continues and expands abnormally, chronic tissue damage is caused. Chronic IBD with repeated recovery and deterioration of the symptoms requires long-term treatment. In the past IBD was regarded to occur only in western people with high meat consumption. However, over the last 10 years it has rapidly increased in many different countries. In Korea, due to the rapid increase of western food consumption, IBD incidence will be similar to that in western countries in a matter of time. Salicylic acid and steroids have been mainly used for IBD treatment. These drugs can cause side-effects, toxicity, and resistance (Choi et al., 2009). Therefore, it is important to consider developing new concepts of anti-inflammatory functional materials for colon disease by employing high value production from natural materials. In addition, colon cancer has increased in Korea as meat consumption has increased with westernized food. Food, gastrointestinal bacteria, and cancer have an important relationship. The consumption of animal fat and a high protein diet boosts micro-organisms in the gastrointestine, which activates β- glucuronidase, tryptophanase, and other enzymes, and it ultimately leads to colon cancer (Choi et al., 1999; Kim et al., 2008). β-glucuronidase is an enzyme that hydrolyses glucuronide units, in which the number 1 carbon of the β-form of glucuronic acid is combined with a glucoside. When a toxic material such as benzo(a)pyrene is detoxified in the liver by glucuronic acid conjugation and sent to the intestine, β-glucuronidase with this compound as a substance is turned into a cancer causing harmful compound by disconnecting combination, which is known to be a harmful enzyme. Tryptophanase is an enzyme that decomposes tryptophan into indol, ammonia, and pyruvic acid. Particularly in high animal protein consumption, tryptophan content is high and indol content in the colon increases. Indol is observed in the intestinal wall, damages kidneys, and causes cystitis. Ammonia, which is produced in damaged cells of the intestine wall, causes changes in nucleic acid combinations, which lead to cancer. Because high pH in the intestine causes activation of β-glucuronidase and tryptophanase, either lowering pH in the intestine or restraining the production of cancer-causing sources by directly removing zymogens is the most recommendable method (Choi et al., 1999; Kim et al., 2008; Rhee et al., 1998; Lee et al., 1995; Shin et al., 1999).
Aloe is a perennial plant and botanically belongs to the Lily family (Liiaceae), and originated in the far south east of Africa. It has been used as an herbal medicine for 3,000 years to enhance stomach function and relaxation. Currently, the leaves of Aloe vera, Aloe arborescence, and Aloe saponaria are used as health functional foods. Aloe gel is known to be obtained from the larger leaves of Aloe vera. Aloe vera gel is prepared as a health functional food by removing the non-edible parts and peeling off the epicarp. The gel is produced for easy consumption by being dried, ground, and concentrated. The main components of aloe vera gel are polysaccharide polymers, alomichin, aloin, aloetic acid, and aloe ulcin, which are known to be effective for antitumor functions, digestion, strengthening immunity, cell reproduction, and improved stomach function (Cho et al., 2006; Hong et al., 1999; Kang et al., 2005; Choi et al., 1996; Lee et al., 1996; Woo et al., 1995; Jeong et al., 1994).
As mushrooms contain a great variety of nutrition, they have been used as food and medicine. Recently, there have been many scientific studies of mushrooms on improving immunity; their anti-cancer, anti-microbial, and antihypertensive effects; lowering of cholesterol; and balancing of blood-sugar levels (Lee et al., 1997; Lee et al., 1998; Lee et al., 1996; Kim et al., 1995; Kim et al., 1991; Hwang et al., 1997; Kim et al., 1998). In particular, a liquid culture method using mushroom mycelium, which enables the production of large amounts of bioactive compounds in a short period of time at lower cost, has been studied for possible utilization on an industrial scale.
Ganoderma lucidum (G. lucidum ), which was used in this study, is in the Polyporaceae of basidiomycetes family and taken as a medicine for diuretic, tonic, immunity, anit-cancer, and mitomycin purposes(Kim et al., 1991; Oh et al., 2005; Bae et al., 2009). It is particularly proven to be very safe through animal experiments of acute and subacute toxicity using extracts from G. lucidum. There are many studies of G. lucidum for its various physiological activities (Kim et al., 1995; Joung et al., 2009; Seo et al., 1996). In addition, Hericium erinaceum (H. erinaceum) has also been used as a food and medicine for a long time since it is effective in chronic gastritis, gastric cancer, anti-cancer functions, and physical weakness. In a recent study, a material for an Alzheimer cure was extracted from H. erinaceum. Furthermore, its hot-water extract has shown an effect on restricting cancer cell proliferation and anti-tumor effects. H. erinaceum contains a β-glucan component, which is known to be effective for immunity and the prevention of arteriosclerosis (Joung et al., 2009; Jeong et al., 2005; Choi et al., 2003; Jung et al., 2006). Phellinus linteus (P. linteus) is effective in improving immunity during chemotheraphy after cancer surgery, especially in gastrointestinal cancers such as stomach cancer, esophageal cancer, duodenal cancer, colon cancer, rectum cancer, and liver cancer. It also has effects on metrorrhagia, leukorrhea, irregular menstruation, enterohemorrhage, improving stomach function, and detoxification.
In this research, three newly-improved functional materials (G. lucidum-aloe-fermented product: AG, H. erinaceum-aloe-fermented product: AH, and P. linteus-aloe-fermented product: AP) of aloe were obtained using G. lucidum, H. erinaceum, and P. linteus. For the usage of aloe-fermented products as a functional material, pH changes during intestinal bacteria cultivation, inhibition activity against β-glucuronidase and tryptophanase, improvements of colitis induced by TNBS, and improvements of gastrointestinal function using female Balb/C mice were examined to evaluate these aloe-fermented products as curative materials for colitis (inflammatory bowel disease).
Materials and Methods
Materials
In this study, Gandorma lucidum (G. lucidum KCTC 6729) and Phellinus linteus (P. linteus KCTC 6719) were used as standard strains and acquired from the Gene Bank of The Korea Biological Resource Center (Daejeon, Korea). Hericium erinaceum (H. erinaceum) was obtained from the Food-Engineering Department of Korea National University of Transportation (Jeungpyeong, Chungbuk, Korea). Each strain was cultivated in potato dextrose agar (PDA, Difco, Sparks, MD, USA) as a medium by 15 days of subculturing (Jeong et al., 2005). Aloe (Aloe vera), for each strain as a substrate material, was purchased from Jeju Samda Aloe (Eoul Eup, Jeju, Korea). To verify improved intestinal function, the enzymes for assessing inhibition activity included β-glucuronidase (G7396) and Tryptophanase (A6007) from Sigma (St. Louis, MO, USA). All reagents used in this experiment were special grade. TNBS (Trinitrobenzene sulfonic acid, Sigma) was used to induce inflammatory bowel disease.
Selection of experimental animals and breeding environment
The animal experiment was performed with permission from The Ethics Committee of Animal Experiments (CBNUR-186-1001) at Chungbuk National University, Korea.
The mice used in this study were six week-old Balb/C female mice (Daehan Bio Link Co., Ltd., Eumseong, Chungbuk, Korea). They went through a quarantine and seven week adjustment period in the animal laboratory. Healthy mice were selected for the experiment, which was performed in the animal laboratory under conditions of 23±3°C, 55±15% relative humidity, 12 hours of lighting (08:00-20:00), and 150-300 Lux of brightness. During the experimental period, the animals were kept in stainless mesh cages (W220×L410×H200 mm). Two to three mice were kept for the adjustment period and 2-3 mice were kept during the injection and observation period. An animal diet for experimental animals (Daehan Bio-Link, 113 Deayari, Samsung, Eumsung, Chungbuk) was given freely. Tap Water disinfected by an ultraviolet sterilizer was provided.
Cultivation conditions for strains and manufacturing of liquid spawn
Manufactured strains of Ganoderma lucidum (all liquid culture) were incubated in PDA medium for seven days at 28°C. The strain discs were created by cutting with a cork borer (Ø 8 mm). Five to six discs were placed in an Erlenmeyer flask which contained 100 mL of potato dextrose broth (PDB, Difco) medium and were then shaken-cultured (SI-400R, Jeiotech Co., Daejeon, Korea) for six days. The culture was homogenized by a grinder (Waring Lab., Torrington, CT, USA). Nine milliliters of this was subsequently placed in an Erlenmeyer flask in which 100 mL of PDB was left for five days. This was used as the main spawn. H. erinaceum was processed in the same method as above but the temperature was kept at 24°C (Jeong et al., 2005).
Cultivation of mushroom mycelium in Aloe as a natural medium
To obtain a viscous aloe gel, fresh leaves were washed, peeled, and ground. The gel was placed in a fermenter and sterilized for 20 minutes at 120°C. The temperature of the fermenter was rapidly cooled to 25°C and this aloe gel was used as a natural medium. Aloe-fermentation products were obtained by cultivating 9% of the liquid spawn in the gel at an optimum temperature. These aloe-fermented products were extracted at 85°C for 6 hours and filtered using filter paper (Advantec 5A, Tokyo, Japan) and then evaporated and freeze-dried (IlShinBioBase Co., Ltd., Dongducheon, Gyeonggi, Korea). The freeze dried aloe fermented products were used as the samples.
pH value changes of the medium culturing intestinal bacteria containing aloe-fermented products
Here, 0.5 and 1.0% (v/v) of aloe-fermented products were added to each 5 mL of GAM medium. The pH was set at 7.2 using 2 N NaOH and sterilized for 15 minutes at 121°C. Fresh feces samples from healthy adults in their 20s were collected for intestinal bacteria and diluted 10-3. A 5 µL amount of the diluted solution was placed on an anaerobic medium and cultivated for 20 hours under anaerobic conditions at 37°C, and pH changes were measured (Rhee et al., 1998; Han et al., 1996).
Inhibition activity against β-glucuronidase
A 0.1 g amount of aloe-fermented product, as a concentrated freeze-dried powder, was dissolved in 10 mL of sterile water. It was filtered with a 0.45 µm membrane filter and then continuously 1/2 diluted, which was used as a sample liquid. β-glucuronidase was diluted with 10 mM sodium phosphate buffer in 400 unit/mL and it was used as the enzyme solution. A 0.02 mL amount of 10 mM ρ-nitrophenyl β-D-glucuronide, 0.38 mL of 10 mM sodium phosphate buffer, and 0.1 mL of sample were added to 0.1 mL of enzyme solution and reacted for one hour at 37°C. The reaction was ended by adding 0.5 mL of 0.5 N NaOH. It was centrifuged (2000×g, 10 min) and the absorbance was measured at 550 nm (Han et al., 1996; Lee et al., 2008).
Inhibition activity against Tryptophanase
Tryptohanase was diluted with 50 mM potassium phosphate (pH 7.5) to 37.5 unit/mL and was used as an enzyme solution. A 0.1 mL amount of the enzyme solution was added to 2.5 mL of the completed reaction mixture [2.75 mg pyrophosphate, 19.6 mg disodium EDTA dihydrate, 10 mg bovine serum albumin/100 mL 0.05 M potassium phosphate (pH 7.5)] along with 0.1 mL of 40 mM tryptophan and 0.1 mL of aloe-fermented products, and then reacted for 30 minutes at 37°C. Two milliliters of color reagent (14.7 g ρ-dimethylaminobenzaldehyde, 52 mL H2SO4) was added and the reaction was finished. It was then centrifuged (2000×g, 10 min) and absorbance was measured at 550 mm (Han et al., 1996; Lee et al., 2008).
The effects on improving inflammatory bowel disease induced by TNBS (Trinitrobenzene Sulfonic Acid)
Six week-old Balb/c female mice were used for this study. Ten milligrams per milliliter of TNBS was resolved in 100% ethanol. The same amount of water was added to the same amount of ethanol and 10 mg of TNBS was resolved in 1 mL of 50% ethanol. The mice were anesthetized by ether. TNBS was injected by a PE90 tube (or polyethylene catheter, 1.5 mm diameter) attached to a syringe through the anus at 4 cm deep (100 µL, 1 mg TNBS). One-hundred microliters of 50% ethanol was injected into the control group using the same method. After TNBS injection, the heads of the mice were kept downward for 1-2 minutes so that the injected TNBS was kept inside and spread evenly within the colon. Next, 62, 185, and 370 mg/kg amounts of the test material were set and injected once every day for seven days before and after colitis induction (refer to arrangement of the experimental group). After 24 hours of test material injection, the colon was collected and cut vertically, washed with saline solution, and weighed. The severity of colitis was assessed using an assessment table (Table 1).
Table 1. Gross morphology of ulcers and inflammatory bowel disease in the colons of experimental animals  |
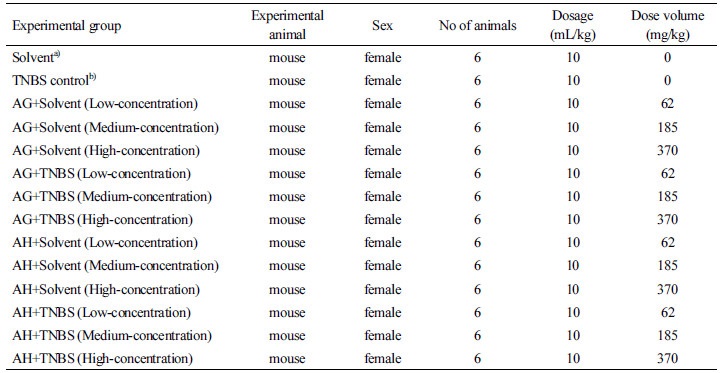
Setting the injection volume and organization of the experimental groups
In this study, the test material was the aloe fermented products. Three different dosages of the test material, 63 mg/10 mL/kg, 185 mg/10 mL/kg, and 370 mg/10 mL/kg were manufactured using distilled water as a vehicle before being administered. No mouse fatalities were observed with 370 mg/kg of the test material during the preliminary experiment; therefore, 370 mg/kg was set as the maximum amount. The vehicle control group was set for vehicle injection. The positive control group was administered 150 mg/kg of Stillen Tab. There are no guidelines provided by the Korea Food and Drug Administration regarding toxicity tests of drugs to provide a dosage limit, so guidelines and references for non-clinical testing of drugs (1997) by the Health and Welfare of Japan were used. The experimental group treatment dosage and the dosage for single-dose toxicity are shown in Table 2. The mouse groups were divided by selecting healthy mice during the adjustment period. They were weighed and separated by 2 g differences. Ten mice close to the average weight were selected. The mice were divided randomly into groups of two or three. The identification of individual mice was performed by color markings on the fur and recognition cards.
Observation and test items
On the day of treatment, any symptoms and mice fatalities were checked every hour for six hours after administration. Body weight and food and water consumption were measured before and after administration. Survived mice that went through every stage of the experiment were anesthetized by ether. Their abodmens were opened and the mice were killed by bleeding. All intestines were observed by the naked eye.
Statistical methods
The SOP test of the Toxicology Institute and the Kruskal Wallis test were performed.
Results and Discussion
Changes of pH value in the medium for culturing intestinal bacteria containing aloe-fermented products
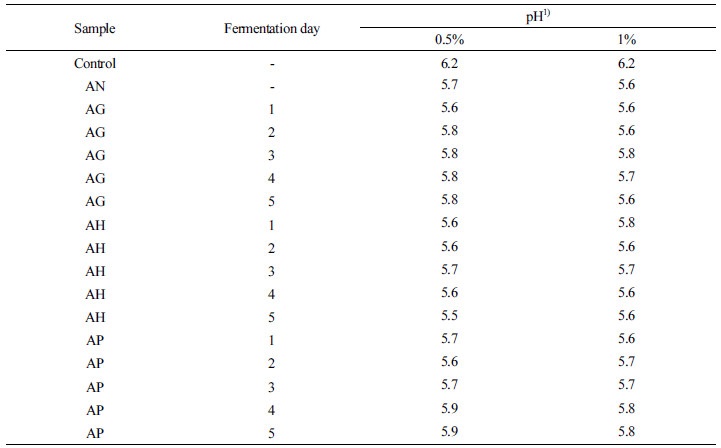
When the aloe-fermented products were added to medium in which intestinal bacteria were cultivated, changes of pH in the medium were investigated. As shown in Table 3, when the medium with aloe-fermented products was combined with intestinal bacteria, the bacteria were well cultivated without exception. In the control group, a pH of 7.2 in the medium before transplanting the intestinal bacteria changed to pH 6.3 after intestinal bacteria cultivation. However, intestinal bacteria cultivation in the medium with aloe-fermented products resulted in a lower pH than the control group. Lowering of pH was more effective with 1% of the aloe-fermented products than with 0.5% of the aloe-fermented products. AH-5 was the most effective material in lowering pH with pH values of 5.5-5.6. The lowering of pH in the medium by these aloe-fermented products would be considered to offer improvements for colitis, such as by lactobacillus which produces much intestinal acid. Aloe-fermented products are considered to contain growth components for intestinal acid-producing bacteria like lactobacillus. Lactobacillus contributes not only to lowering of pH, but also inhibits the production of harmful enzymes such as β-glucronidase and tryptophanase (Kim et al., 1995).
Inhibition effects against β-glucuronidase and tryptophanase
In β-glucuronidase inhibition, all aloe-fermented products showed inhibition of β-glucuronidase activity, whereas the control group (AN) showed only 15.8±4.8% of β-glucuronidase inhibition. For AG, initially 0.5 mg and 1.0 mg of AG were added, but regardless of the amount added or the fermentation days, AG showed a minimum of 56% β-glucuronidase inhibition, which was the most effective. AH showed greater variation compared to AG by days of cultivation. On the 4th day of cultivation, 0.5 mg and 1 mg were added, and 42±5.4% and 45± 2.1% of β-glucuronidase was inhibited, respectively. In AP, inhibition increased to a maximum of 46% in 1-3 days of cultivation. However, inhibition decreased with longer days of cultivation. Yet, AP remained a maximum 3 times more effective than the control group (Fig. 1A).
In tryptophanase inhibition, whereas the control group (AN) showed 7.0± 2.0%, all experimental groups with the addition of 1 mg of the aloe-fermented product showed a minimum 24% improved effectiveness. When the amount of added aloe-fermented product decreased from 1 mg to 0.5 mg, inhibition of tryptophanase also decreased. In AG, a maximum 72% inhibition was shown for four days of cultivation, but inhibition decreased on the fifth day of cultivation. In AH, for five days of cultivation, from the first day (40±5.3%) to the fifth day (76± 4.5%) inhibition of tryptophanase increased as cultivation days increased. In AP, no pattern was shown with the cultivation period; however, more effective inhibition was shown compared to that in the control group (AN) (Fig. 1B).
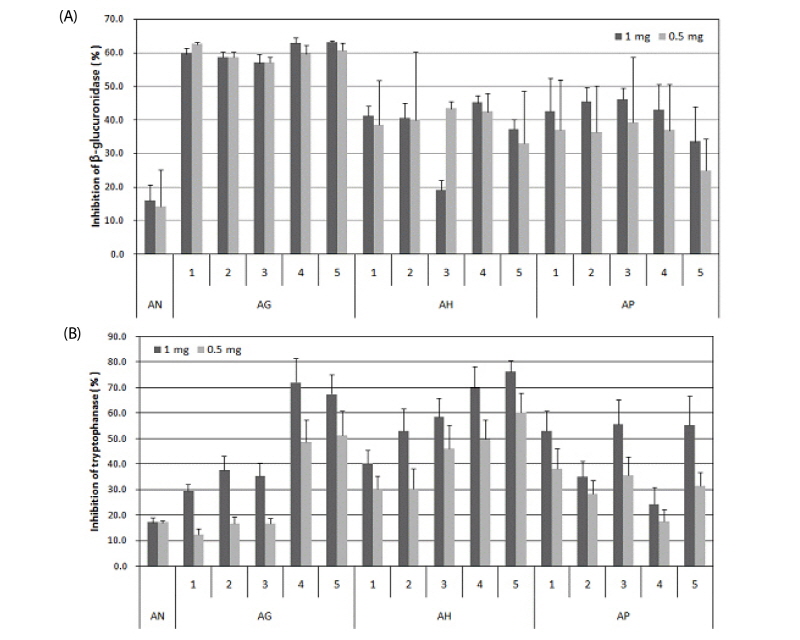
Fig. 1.
Inhibition effects of aloe fermented products against β-glucuronidase(A) and tryptophanase(B).
*AN : Natural Aloe vera, *AG: Fermented product of Ganoderma lucidum in aloe substrate, *AH: Fermented product of Hericium erinaceum in aloe substrate, *AP: Fermented product of Phellinus linteus in aloe substrate.
According to the above results, considering the stability and effectiveness of inhibition against β-glucuronidase and tryptohanase by these aloe-fermented products, AG-4 and AG-5 were the most effective for industrialization of a product.
Improvement effects on inflammatory bowel disease (IBD) induced by trinitrobenzen sulfonic acid (TNBS) in a mouse model
Fatality rate and LD50 values
There were no fatalities in the vehicle control group, and the test material group was administered with 370 mg/kg during the experiment period. The LD50 value of the test material was considered to be over 370 mg/kg.
Gross morphology induced by TNBS
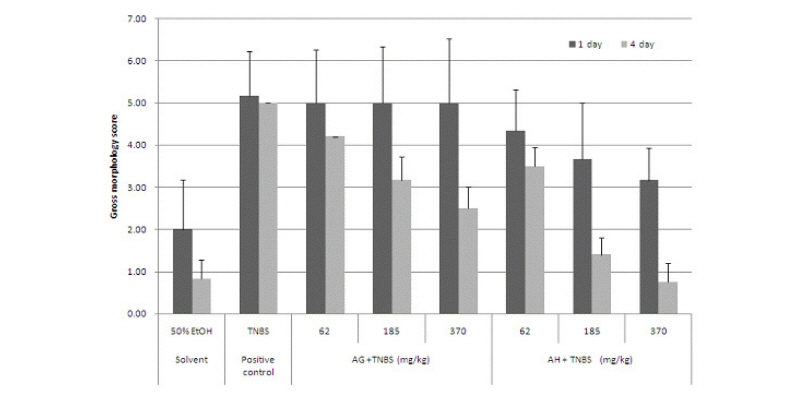
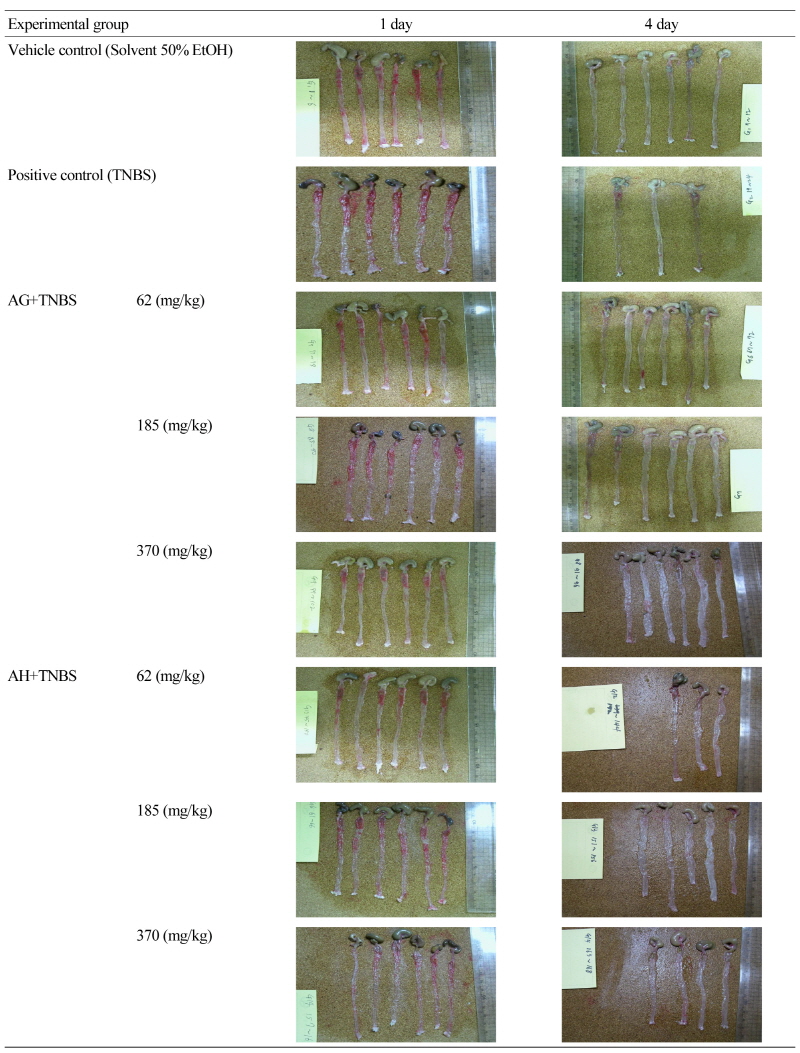
The test material was administered orally in three different dosages once every day for a week before TNBS was administered. The mice were fasted one day before TNBS administration. The colon was collected after 24 hours and 4 days of TNBS administration, and cut vertically and washed with saline solution. The severity of colitis was assessed with an assessment table and calculated by scores (Table 1). AG did not show any effects on colitis induced by TNBS with the administration of AG one week before TNBS administration. AH showed a slight preventative effect on colitis induced by TNBS (Fig. 2). Both AG and AH showed relief effects for colitis when administered continually after inducing colitis by TNBS. The colon was collected after 24 hours and 4 days and observed with the naked eye following the induction of colitis in the Balb/C mice with TNBS. The colons of the mice with colitis induced by TNBS were shortened compared to the vehicle control group and congestion on the colon was observed. The groups that received the aloe-fermented products showed improvements of colon damage induced by TNBS similar to the normal groups (Fig. 6).
Fig. 2.
Gross morphology of ulcers and inflammatory bowel disease in the colons of experimental animals.
50% EtOH: Vehicle control group (50% Ethanol solvent), TNBS: Positive group administered TNBS, AG+TNBS (62 mg/kg): Sample group administered 62 mg/kg AG and TNBS, AG+TNBS (185 mg/kg): Sample group administered 185 mg/kg AG and TNBS, AG+TNBS (370 mg/kg): Sample group administered 370 mg/kg AG and TNBS, AH+TNBS (62 mg/kg): Sample group administered 62 mg/kg AH and TNBS, AH+TNBS (185 mg/kg): Sample group administered 185 mg/kg AH and TNBS, AH+TNBS (370 mg/kg): Sample group administered 370 mg/kg AH and TNBS.
AG: Fermented product of Ganoderma lucidum in aloe substrate, AH: Fermented product of Hericium erinaceum in aloe substrate.
Body weight changes
Body weight was measured for five days of injection with TNBS and administration of the aloe-fermented products using a digital mass meter. The vehicle control group, TNBS treatment group, test material AG+TNBS treatment group, and test material AH+TNBS treatment group were weighed. The vehicle control group and the TNBS treatment group showed decreases in weight, which is a typical symptom of inflammatory bowel disease. The TNBS treatment group showed significant weight loss near the end of the experiment. However, the groups that received the aloe-fermented products AG and AH showed recovery from weight loss induced by TNBS (Fig. 3).
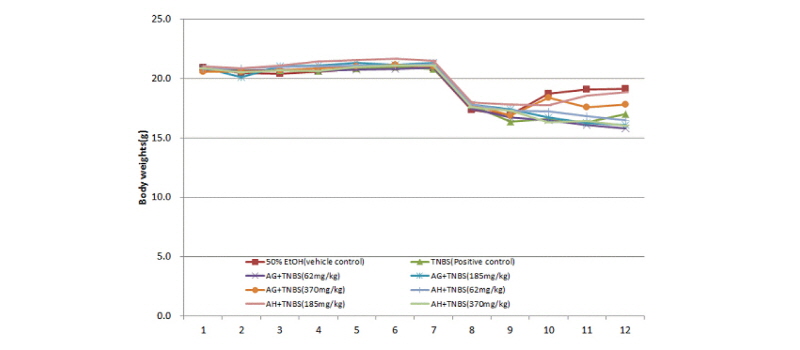
Fig. 3.
Body weight changes in Balb/c mice against an inflammatory bowel disease induced by a trinitrobenzene sulfonic acid solution.
50% EtOH: Vehicle control group (50% Ethanol solvent), TNBS: Positive group administered TNBS, AG+TNBS (62 mg/kg): Sample group administered 62 mg/kg AG and TNBS, AG+TNBS (185 mg/kg): Sample group administered 185 mg/kg AG and TNBS, AG+TNBS (370 mg/kg): Sample group administered 370 mg/kg AG and TNBS, AH+TNBS (62 mg/kg): Sample group administered 62 mg/kg AH and TNBS, AH+TNBS (185 mg/kg): Sample group administered 185 mg/kg AH and TNBS, AH+TNBS (370 mg/kg): Sample group administered 370 mg/kg AH and TNBS.
AG: Fermented product of Ganoderma lucidum in aloe substrate, AH: Fermented product of Hericium erinaceum in aloe substrate.
Food consumption and water consumption
The group with colitis induced by TNBS showed a significant decrease in food and water consumption on the second and third days. However, food and water consumption increased after the third day, but the increase was no more than 20%. For the issue of diarrhea caused by colitis, no diarrhea was observed before colitis was induced. Diarrhea, which is a typical symptom of colitis, was observed after colitis was induced and became severe in the control group. In the Balb/C mice where colitis was induced by TNBS, diarrhea worsened as time lapsed, a chronic colitis symptom, along with blood in the diarrhea. The groups that received the aloe-fermented products showed little diarrhea and recovered from the first day of test material intake. In particular, the AG and AH groups showed stable conditions for diarrhea, maintaining a consistent pattern after the second day of test material administration (Fig. 4 and 5).
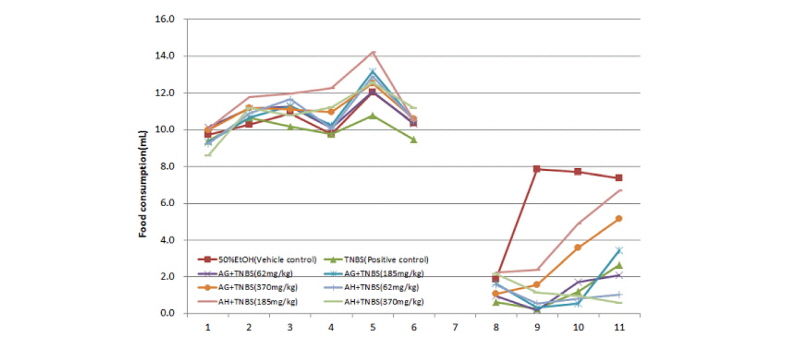
Fig. 4.
Food consumption in Balb/c mice with inflammatory bowel disease induced by a trinitrobenzene sulfonic acid solution.
50% EtOH: Vehicle control group (50% Ethanol solvent), TNBS: Positive group administered TNBS, AG+TNBS (62 mg/kg): Sample group administered 62 mg/kg AG and TNBS, AG+TNBS (185 mg/kg): Sample group administered 185 mg/kg AG and TNBS, AG+TNBS (370 mg/kg): Sample group administered 370 mg/kg AG and TNBS, AH+TNBS (62 mg/kg): Sample group administered 62 mg/kg AH and TNBS, AH+TNBS (185 mg/kg): Sample group administered 185 mg/kg AH and TNBS, AH+TNBS (370 mg/kg): Sample group administered 370 mg/kg AH and TNBS.
AG: Fermented product of Ganoderma lucidum in aloe substrate, AH: Fermented product of Hericium erinaceum in aloe substrate.
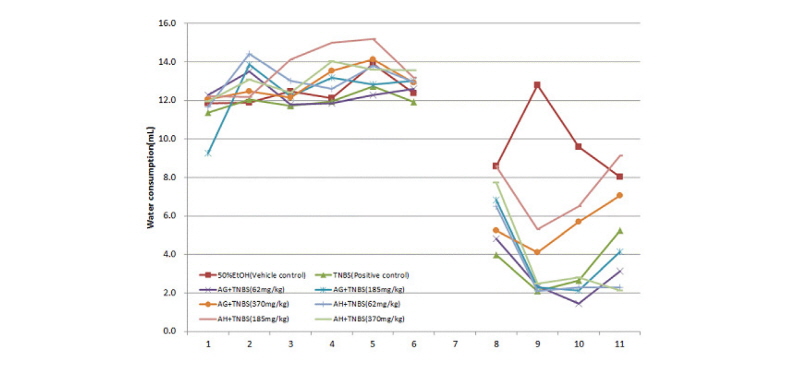
Fig. 5.
Water consumption in Balb/c mice with inflammatory bowel disease induced by a trinitrobenzene sulfonic acid solution.
50% EtOH: Vehicle control group (50% Ethanol solvent), TNBS: Positive group administered TNBS, AG+TNBS (62 mg/kg): Sample group administered 62 mg/kg AG and TNBS, AG+TNBS (185 mg/kg): Sample group administered 185 mg/kg AG and TNBS, AG+TNBS (370 mg/kg): Sample group administered 370 mg/kg AG and TNBS, AH+TNBS (62 mg/kg): Sample group administered 62 mg/kg AH and TNBS, AH+TNBS (185 mg/kg): Sample group administered 185 mg/kg AH and TNBS, AH+TNBS (370 mg/kg): Sample group administered 370 mg/kg AH and TNBS.
AG: Fermented product of Ganoderma lucidum in aloe substrate, AH: Fermented product of Hericium erinaceum in aloe substrate.
Length, weight, and shape of colon
To determine symptoms in the colons with induced colitis, the colons were collected by anesthesia with ether after 24 hours and 4 days of injections of the colitis inducing material, and were cut open. The shapes, weights, and lengths of the colons in the groups receiving the test material, or aloe-fermented products, were in good condition compared to the vehicle control group (Fig. 6). This pattern coincided with weight changes and food and water consumption patterns. For the shapes of the colons, the control group (50% ethanol) showed severe hypertrophy of the colon wall, a thinner intestinal wall, and many ulcers and congestion, which shortened the colon and made the colon heavier so that it became adhesive to other organs that were adjacent to the colon such as the small intestine and stomach. When the colons were also cut open, phathological changes (visual lesions) such as ulcers and colitis conditions were scored from 0 to 10 by inflammation of the colon, ulcer existence, and size of ulcers, and were scored as a set. Through the IBD test model induced by TNBS, the experimental groups that received the aloe-fermented products showed decreases in colon mucosa damage and severity of colitis compared to the control group (Fig. 6).
General opinion
The main area of lesions in IBD is in the mucosa and lower mucosal, and IBD often appears with colitis and ulcers. IBD starts from the rectum and gradually spreads upward and reaches to the colon. The most commonly used drugs for IBD are salicylic acid and steroids, which have 80% effectiveness in curing ulcerous colitis. Currently, reoccurrence of IBD is 50% with the most effective method, and most drugs have been restricted for their side-effects. Therefore, the development of new drugs and methods is one of the main issues for IBD treatment. Due to limited clinical experiments on human beings for studying causes of the disease, pathogenesis using animal models has been the main means of drug development. An IBD model using acetic acid induces acute inflammation and provides easy observation for the effects of model drug treatments. Because of similarities to human IBD symptoms physiological to mice, and the induction of chronic inflammation, an IBD mouse model using trinitrobenzene sulfonic acid (TNBS) is commonly used to study the effectiveness of drugs along with mechanisms. Therefore, in this study, the possibility of aloe-fermented products as treatment material for gastrointestinal improvement was assessed using a commonly employed animal model by inducing IBD and administering aloe-fermented products. The IBD mouse model used in this study with administration of TNBS showed that the tested aloe-fermented products had significantly better effects as compared to the vehicle control group. Particularly, since the assessments of the severity of colon damage showed there were satisfactory improvements, aloe-fermented products have viability in term of development as treatment materials for ulcerous colitis, also known as inflammatory bowel disease. According to these results, aloe-fermented products can be considered treatment materials for gastrointestinal improvement.
Summary
Aloe-fermented products (G. lucidum-aloe-fermented product: AG, H. erinaceum-aloe-fermented product: AH and P. linteus-aloe-fermented product: AP) were obtained by fermenting Ganodorma lucidum, Hericium erinaceum, and Phellinus linteus with aloe during a fermentation period. In this study, the aloe-fermented products were examined in order to determine their effects on gastrointestinal function improvement. First, in a medium in which intestinal bacteria were cultivated with the aloe-fermented products, there was an improved effect on lowering pH than in the control group without the aloe-fermented products. Adding more aloe-fermented product from 0.5% to 1.0% further lowered pH. Sample AH-5 was the most effective, lowering pH by 1.7.
For inhibition effects against β-glucuronidase and tryptophanase, β-glucuronidase was significantly inhibited by all the aloe-fermented products compared to the control group (AN) which showed 15.8±4.8% inhibition. When 0.5 mg and 1 mg of AG were added, regardless of the fermentation period and amount of AG, the inhibition effect of AG was a minimum of 56%, which was very significant. Furthermore, when 0.5 mg and 1 mg of AH were added with four days of cultivation, they showed inhibitions 42±5.4% and 45±2.1%, respectively, which were stable. AP with one to three days of cultivation showed an increase in inhibition with a maximum of 46%. For inhibition effects on tryptophanase, the test groups with 1 mg of the aloe-fermented products showed better inhibition effects than the control group (AN), which showed 17.0±2.0%. When the amount of aloe-fermented product was reduced from 1 mg to 0.5 mg, the inhibition effect was decreased. AG showed a maximum of 72% inhibition with four days of cultivation. AH showed a maximum of 76±4.5% with five days of cultivation. AP showed no stable pattern with the cultivation period; however, it did at least show better inhibition effect than the control group (AN). Therefore, considering the stability and effectiveness of their inhibition effects against β-glucuronidase and tryptophanase, AG-4 and AG-5 are products to consider for industrialization.
In order to determine their effects on improvement of inflammatory bowel disease, AG and AH were administered to Balb/c mice for five days after TNBS injection. The test material AG, which was administered a week before colitis induction by TNBS, did not affect colitis induction. The test material AH, however, showed a slight preventive effect on colitis induction. AG and AH were also administered continually after inducing colitis and alleviation of colitis was shown. Therefore, aloe-fermented products can be considered functional foods for gastrointestinal improvement, especially for the improvement and healing of inflammatory bowel disease.
Symbols and Terminology
* AN : Natural Aloe vera
* AG : Fermented product of Aloe by the cultivation of G. lucidum
AG-1: Fermented product of Aloe by the cultivation of G. lucidum for 1 day
AG-2 : Fermented product of Aloe by the cultivation of G. lucidum cultivated for 2 days
AG-3 : Fermented product of Aloe by the cultivation of G. lucidum cultivated for 3 days
AG-4 : Fermented product of Aloe by the cultivation of G. lucidum cultivated for 4 days
AG-5 : Fermented product of Aloe by the cultivation of G. lucidum cultivated for 5 days
* AH : Fermented product of Aloe by the cultivation of H. erinaceum
AH-1 : Fermented product of Aloe by the cultivation of H. erinaceum for 1 day
AH-2 : Fermented product of Aloe by the cultivation of H. erinaceum for 2 days
AH-3 : Fermented product of Aloe by the cultivation of H. erinaceum for 3 days
AH-4 : Fermented product of Aloe by the cultivation of H. erinaceum for 4 days
AH-5 : Fermented product of Aloe by the cultivation of H. erinaceum for 5 days
* AP : Fermented product of Aloe by the cultivation of P. linteus
AP-1 : Fermented product of Aloe by the cultivation of P. linteus for 1 day
AP-2 : Fermented product of Aloe by the cultivation of P. linteus for 2 days
AP-3 : Fermented product of Aloe by the cultivation of P. linteus for 3 days
AP-4 : Fermented product of Aloe by the cultivation of P. linteus for 4 days
AP-5 : Fermented product of Aloe by the cultivation of P. linteus for 5 days