Introduction
Materials and methods
Materials
Preparation of WWE
Determination of AX contents
MI Experiments
AD Experiments
Western blotting
Statistical analyses
Results and Discussion
Wheat varieties and WWE preparation
Comparison of efficacy between three WWEs using MI model
Efficacy of Anbaek WWE at various doses in MI model
Correlation between AX content and efficacy in MI model
Efficacy and underlying mechanisms of Anbaek WWE in AD model
Introduction
Geriatric disorders are age-related diseases, incidence of which increases as people age. The disorders include cardiovascular disease and neurological disorders that result from death of cells constituting the corresponding organs (Lim et al., 2018) (Fig. 1). Cardiovascular disease, the leading cause of mortality worldwide, includes blood vessel diseases of the heart such as angina pectoris, myocardial infarction (MI), and heart failure. When the coronary arteries that supply blood to the heart narrow by accumulation of atherosclerotic plaques, angina pectoris occurs due to insufficient supply of blood to the heart. Atherosclerotic plaques occasionally rupture, then thrombus forms in the lesion, subsequently the artery is occluded. As a result, myocardial cells die extensively through apoptosis and necrosis due to insufficient production of ATP, leading to MI (Kim et al., 2017). MI in turn triggers adverse remodeling in the heart, leading to heart failure in which the heart cannot supply sufficient amount of blood to the whole body (Lim et al., 2016). Cardiovascular disease also includes blood vessel diseases of the brain such as ischemic and hemorrhagic strokes . Ischemic stroke is caused by occlusion of cerebral arteries and subsequent death of brain cells in the lesion. MI and ischemic stroke are two major subtypes of ischemic diseases.
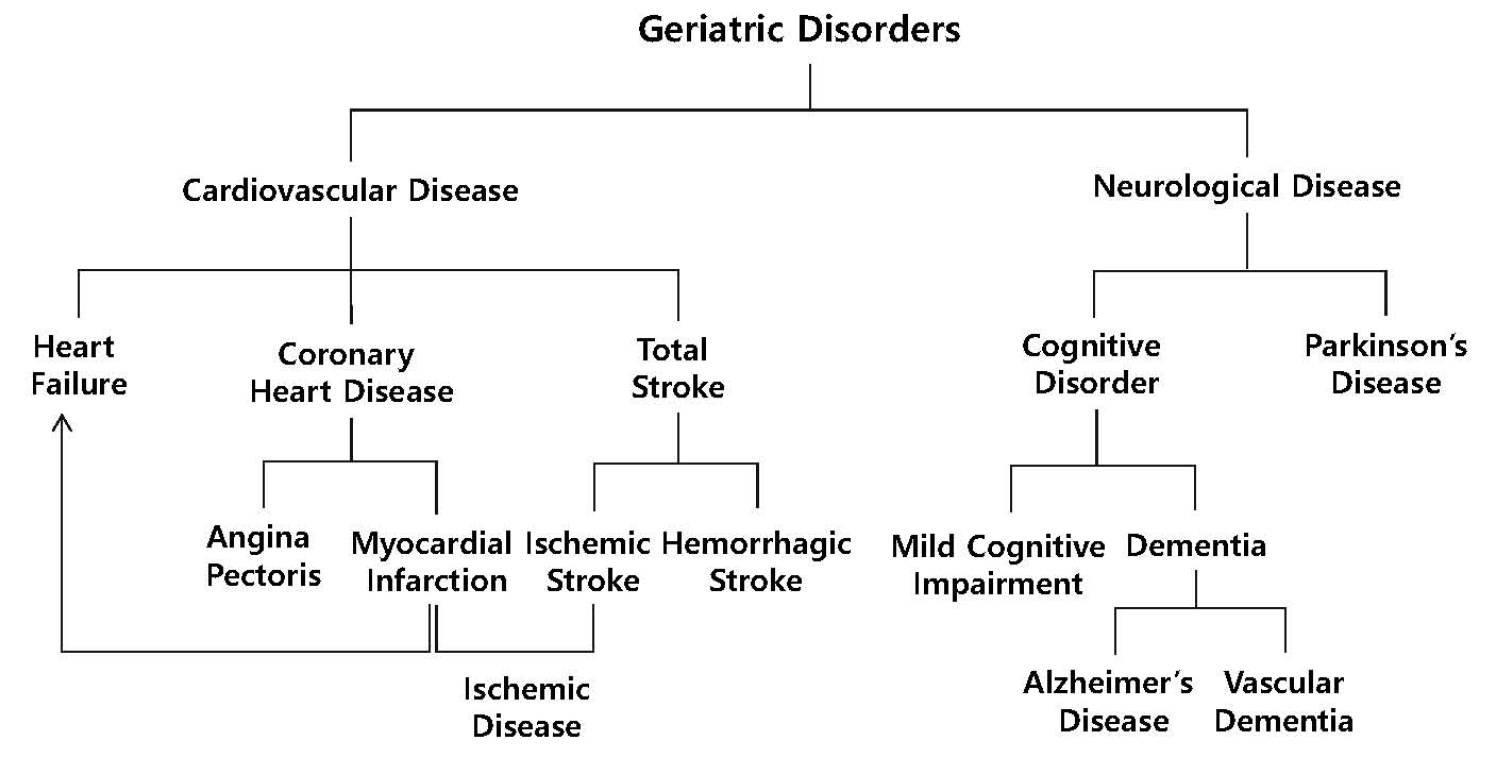
Fig. 1.
Classification of geriatric disorders.
Geriatric disorders include cardiovascular diseases (CVDs) and neurological diseases (NDs). CVDs include coronary heart disease, heart failure, and total stroke. Angina pectoris and myocardial infarction are classified as coronary heart diseases, while ischemic and hemorrhagic strokes are classified as total strokes. Among these CVDs, myocardial infarction and ischemic stroke are regarded as the two major forms of ischemic diseases. NDs include cognitive disorders and Parkinson’s disease. Cognitive disorders, in turn, include mild cognitive impairment and dementia. Alzheimer’s disease and vascular dementia are two major forms of dementia. The figure was modified after Lim et al. (2018).
On the other hand, neurological disorders include cognitive disorder, Parkinson’s disease, and multiple sclerosis, of which cognitive disorders including mild cognitive impairment and dementia are the most common. While patients with mild cognitive impairment can live normally, patients with dementia need daily care due to severe cognitive deficit. Of dementia, Alzheimer’s disease (AD) is the most common type of dementia accounting for approximately 60-70% occurrence, followed by vascular dementia (VaD) (Kalaria, 2018; Maclin et al., 2019). VaD develops from insufficient blood supply to white matter, leading to lesions in specific white matter regions (Kalaria, 2018). In contrast, AD progresses from synaptic dysfunction to the neuronal loss in the neocortex including hippocampus caused by overproduction of amyloid beta and hyperphosphorylation of tau protein (Jackson et al., 2019). Neurotransmission required for proper cognitive function occurs at synapses where neurotransmitters such as glutamate and acetylcholine are released from presynaptic neurons to synaptic clefts, and then neurotransmitters released bind to the corresponding receptors located on the cell membranes of postsynaptic neurons, leading to generation of signal transduction and action potential (Ho et al., 2011; Sudhof, 2018). For example, scopolamine blocks cholinergic neurotransmission by binding to binding sites for acetylcholine on muscarinic acetylcholine receptors, resulting in amnesia (Hasselmann, 2014; Lakstygal et al., 2019). Synaptic dysfunction in AD occurs when neurotransmission is dysregulated at synapses, including improper release of neurotransmitters from synaptic vesicles and impairment in the interaction between neurotransmitters and the corresponding receptors (Chen et al., 2019; Pei et al., 2020). In the early stage of AD, for example, acetylcholine level in the hippocampus is reduced by impaired release of acetylcholine from presynaptic cholinergic neurons, leading to dysregulated cholinergic neurotransmission and consequent cognitive deficit (Douchamps and Mathis, 2017; Hampel et al., 2018). Based on these results, acetylcholinesterase inhibitors including donepezil that can increase acetylcholine level in the synaptic cleft by inhibiting acetylcholine degradation were developed as drugs to treat AD (Kabir et al., 2019).
Common wheat (Triticum aestivum L.) is one of three major crops including maize and rice. Grains of wheat consist of germ, endosperm from which wheat flour is obtained, and bran which is the multi-layered outer skin of the edible kernel. Endosperm consists of cells which contain starch granules and proteins. The starch granules and proteins are surrounded by cell walls consisting mostly of cell wall polysaccharides. On the other hand, the bran consists mostly of cell wall polysaccharides (Shewry et al., 2013). The cell wall polysaccharides are dietary fiber which is not digested in the small intestine by human but can be fermented by microorganisms inhabiting the large intestine. Of the cell wall polysaccharides of wheat, arabinoxylan (AX) consisting of arabinose and xylose is the major polysaccharide. AX can be divided into water-extractable AX (WE-AX) and water-unextractable AX (WU-AX), depending on water solubility (Gebruers et al., 2008; Saulnier et al., 2012). In our previous studies, to increase WE-AX content from wheat grains we first prepared wheat-extract powder (WE) by grinding whole wheat grains, extracting the ground wheat in boiling water, centrifuging, concentrating, and freeze-drying the extract (Jang et al., 2010). Then we showed using rodents that WE was effective in the models of MI and heart failure (Lim et al., 2016), ischemic stroke (Han et al., 2008), AD (Jang et al., 2010), and VaD (Han et al., 2010). In addition, we also showed that AX, arabinose, and xylose were active components in models of MI (Lim et al., 2016) and AD (Kim et al., 2014). We found that it was difficult to remove starch in large-scale WE production once starch granules were gelatinized by boiling. To solve this problem, we produced wheat-bran extract powder (WBE) in large scale by removing starch granules through centrifugation in cold water, then extracting the remnant wheat bran in boiling water, filtering, concentrating, and spray drying the extract (Lim and Lee, 2014). Then we showed that WBE was effective in the models of AD (Lee et al., 2015) and VaD (Lim and Lee, 2014). Based on efficacies observed for animal studies, we conducted a clinical trial for elderly complaining subjective memory impairment. We found from the clinical trial that cognition in the domains related to vision was improved after the subjects ingested WBE 3 g/day for 3 months (Choi et al., 2018). These results suggest that wheat can be developed as a functional food to prevent mild cognitive impairment and even dementia. As described above, WBE production, however, required complicated production procedures and consequent high production cost, preventing WBE supply in a large amount. To overcome problems occurring in WBE production, it is crucial to develop production protocols not requiring centrifugation or filtration.
In this study, we tested whole-wheat extract powder (WWE) prepared from three Korean wheat varieties using animal models of MI and scopolamine-induced AD to correlate the lowest dose with the highest WE-AX content in the wheat flour of the varieties. Based on these results, we chose Anbaek the final candidate to be developed as a functional food, and proposed a plausible mechanism of Anbaek WWE in improving memory from viewpoint of synaptic neurotransmission.
Materials and methods
Materials
Of three wheat varieties tested in this study, Anzunbaengi was purchased from a grower (Jinju, Gyeongsangnam-do, Korea), and Anbaek and Goso were provided by National Institute of Crop Science, Rural Development Administration (RDA) (Wanju, Jeollabuk-do, Korea).
Preparation of WWE
WWE was prepared through grinding, extraction and drying as described previously, with a modification (Jang et al., 2010). Whole wheat grains (100 g) were ground in a mixer (FoodMixer, Hyun Dae Household Appliances Co., Ltd., Incheon, Korea) for 5 min. The ground grains were extracted with 10-fold dilutions of boiling water in an extractor (DW-290, DaewoongBio.Net, Goesan, Chungcheongbuk-do, Korea) for 1 h. The whole extract was dried at 60°C in a drying oven (Daihan Scientific Co., LTD, Wonju, Ganwon-do, Korea).
Determination of AX contents
Contents of WE-AX and WU-AX in the wheat flour was determined using a phloroglucinol colorimetric assay as described previously (Douglas, 1981; Kang et al., 2013). For the extraction of WE-AX, wheat flour (125 mg) and distilled water (25 mL) were added into 50 mL tubes, stirred for 30 min, and centrifuged at 3000 rpm for 30 min. For WE-AX determination, supernatant (2 mL) was transferred to 50 mL tubes. For WU-AX determination, precipitate (5 mg) and distilled water (2 mL) were transferred to 50 mL tubes. To get standard curve, 10 mg D-(+)-xylose was dissolved in distilled water (100 mL). The xylose solution (0, 0.5, 1.0, 1.5, and 2.0 mL) was transferred to 50 mL tubes, and appropriate volume of distilled water was added into each tube to make final volume of 2 mL in each tube. For the colorimetric assay, colorimetric solution was prepared by mixing acetic acid (110 mL), hydrochloric acid (2 mL), and phloroglucinal [20%(w/v) dissolved in ethanol (5 mL)], and glucose [1.75%(w/v) dissolved in distilled water]. Colorimetric solution (10 mL) were added into each extraction tube and xylose tube, and stirred well with pipettes. Color reaction proceeded in a boiling water bath for 25 min, and the reaction was terminated by putting the tubes in an ice box. Absorbance was measured at 510 nm and 552 nm. Standard curve for xylose was obtained by subtracting absorbance at 510 nm from absorbance at 552 nm. Absorbance for WE-AX and WU-AX was calculated by subtracting absorbance at 510 nm from absorbance at 552 nm, and the content of WE-AX and WU-AX in the wheat flour was calculated using standard curve.
MI Experiments
Eight-week-old male Sprague-Dawley (SD) rats were purchased from Samtaco, Inc (Osan, Gyeonggi-do, Korea). The experiments were performed in accordance with the guidelines for animal care and use of laboratory animal protocols approved by the Institutional Animal Care and Research Advisory Committee of Daegu Catholic University, Daegu, Korea (Approval number: DCIAFCR-200518-10). The animals were housed with rodent food and water available ad libitum under diurnal lighting conditions in a temperature-controlled environment until the experiment began.
Diets containing WWE were prepared and administered as described previously (Lim and Lee, 2017; Lim et al., 2016). The rats were randomly assigned to one of the 5 groups and acclimatized for 3 days with rodent food purchased from Raon Bio Co., LTD (Yongin, Gyeonggi-do, Korea): (1) control (n=8) and (2) WWE-treated groups (31, 63, 125, and 250 mg/kg per day) (n=5, 5, 5 and 5, respectively). In the WWE-treated groups, the rats (~ 300 g) received 15 g/day of the 31, 63, 125, and 250 mg/kg per day WWE diet for 3 days before ligation. After the rats consumed all of the WWE diets, additional rodent food was provided ad libitum. In the control groups, the rats received the rodent food only.
Myocardial injury was generated through ischemia (30 min)/reperfusion (3 h) (I/R) using ligation of the left anterior descending artery (LAD) and subsequent release of the ligation as described previously (Lim and Lee, 2017; Lim et al., 2016). The male SD rats (~300 g) were anesthetized through intramuscular injections of ketamine (100 mg/kg) and xylazine (5 mg/kg), intubated, and ventilated with air throughout the experiment. The heart was then exposed by a left thoracic incision, and the LAD of the rats in the control and WWE-treated groups was ligated for 30 min approximately 5 mm distal to the aortic origin. Ligation was achieved with a 5-0 Prolene suture (BV-1, Ethicon, Somerville, NJ, USA) that was double-knotted. Occlusion generated by the ligation was confirmed by observing the development of a pale color in the left ventricular wall. Subsequently, the heart was reperfused for 3 h via the release of the ligation. During surgery, the rectal temperature was maintained at 37 ± 0.5°C using a thermostat-controlled warming plate (Harvard Apparatus, Holliston, MA, USA). After the I/R procedure, infarct size was assessed using 2,3,5-triphenyltetrazolium chloride (TTC) staining as follows; The LAD was ligated again, followed by the infusion of 1 ml of 1% Evans blue dye (SigmaAldrich, St. Louis, MO, USA) into the heart through the jugular vein. Next, the rats were euthanized by thoracotomy under anesthesia. The heart was harvested, excised into 4 pieces each approximately 3 mm thick, and the pieces were stained with TTC. The area at risk (AAR) was defined as the area that was not infiltrated by Evans blue dye. The infarct area (IA) was defined as the area that was not stained with TTC. The AAR, IA, and left ventricular area (LVA) were determined by computerized planimetry using ImageJ software (NIH, v1.47). Then, the IS and risk size (RS) were calculated using the areas, which were defined as the percentage of the IA to the AAR and the AAR to the LVA, respectively.
AD Experiments
Four-week-old, male ICR mice were purchased from DBL (Eumseong-gun, Chungcheongbuk-do, Korea). Experiments were carried out according to the protocols for animal care and use of laboratory animals, which were approved by the Institutional Animal Care and Use Committee of Daegu Haany University, Gyeongsangbuk-do, South Korea (Approval number: DHU2017-084). Until the start of the experiment, the animals were housed with rodent food and water ad libitum, under diurnal lighting conditions, and in a temperature-controlled environment. Animals were randomly classified into 8 groups (n=9): (1) normal (Nor), (2) control (Con), (3) WWE-treated (31, 63, 125, 250, and 500 mg/kg), and (4) donepezil-treated (positive control) (DNPZ) groups. WWE dispersed in saline (WWE-treated group), donepezil hydrochloride (SigmaAldrich, St. Louis, MO, USA) dissolved in saline (5 mg/kg) (donepezil-treated group), and saline (normal group and control group) were orally administered 1 h before the test. Scopolamine hydrobromide (SigmaAldrich, St. Louis, MO, USA) (1 mg/kg) was intraperitoneally injected 30 min after WWE (WWE-treated group), donepezil (donepezil-treated group), and saline administration (control group) except normal group in which saline was intraperitoneally injected after saline administration.
Y-maze test was conducted to test spontaneous alternation as described previously (Lee et al., 2021a). Y-maze is composed of 3 black arms with 120° (42 × 3 × 12 cm). A mouse was located in one arm and monitored arm entrances for 8 min. When the mouse entered 3 different arms serially, we called it 1 actual alternation. Spontaneous alternation was calculated using the following formula: spontaneous alternation (%) = actual alternation/maximum alternation x 100 (maximum alternation = total entry – 2).
Passive avoidance test was conducted to test step-through latency as described previously (Lee et al., 2021b). Passive avoidance box is composed of two compartments including dark and illuminated compartments, which are separated with guillotine door. In a training session, a mouse was located in illuminated compartment and then guillotine door was opened 10 sec later. When the mouse cross the guillotine door and enter the dark compartment, the door was closed and foot shock (0.5 mA, 3 sec) was delivered through grid floor. At the next day, the mouse was re-located in the illuminated compartment and then guillotine door was opened 10 sec later. Latency time to enter the dark compartment was measured by indicated duration (300 sec).
Western blotting
Western blotting was performed as previously described (Lim et al., 2016). Mice were sacrificed and their hippocampi were extracted rapidly. The hippocampi were homogenized with radioimmunoprecipitation assay (RIPA) buffer (Cell Signaling, Beverly, MA, USA) containing protease inhibitor cocktail. Protein extracts in the supernatant were separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels. The proteins on the gels were transferred to polyvinylidene (PVDF) membranes (Bio-Rad Laboratories, Inc, Hercules, CA, USA) and blocked with 5% skim milk prior to incubation with each primary antibody. The primary antibodies used were phosphorylated synapsin 1 (p-SYN1), PSD95, and GluN2B, a subunit of N-methyl-D-aspartate (NMDA) (1:1000, Cell Signaling, Beverly, MA, USA), neuroligin 3 (NL3) (1:1000, Abcam, Cambridge, MA, USA), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:2000, Santa Cruz Biotechnology Inc, Santa Cruz, CA, USA). In addition, horseradish peroxidase-labeled secondary antibodies (1:2000, Enzo, Farmingdale, NY, USA) were used. The membranes were developed with electrochemoluminescent (ECL) substrate solution (Thermo Fisher Scientific, Rockford, IL, USA), using the ImageQuant LAS 4000 mini (GE Healthcare Bio-Sciences AB, Uppsala, Sweden). The intensities of the protein bands were quantified using ImageJ software (NIH, v1.47). In the quantitative analysis, ratios between various combinations of proteins and phosphorylated proteins were presented using GAPDH as a loading control by setting the control group's value (0 mg/kg of WWE) at 1.
Statistical analyses
The data are expressed as the mean ± standard error of the mean (SEM). All of the statistical analyses were performed in GraphPad prism 7. One-way ANOVA was used for statistics and Newman-Keuls test or Fisher’s LSD test was used to confirm the significance between groups (p < 0.05).
Results and Discussion
Wheat varieties and WWE preparation
Korean wheat varieties investigated in this study were Anbaek containing relatively high WE-AX content (Kang et al., 2013), Anzunbaengi, a native Korean germplasm (Heo et al., 2013), and Goso cultivated widely in Korea for cookies (Kang et al., 2015). Yield of all WWEs prepared through grinding, extraction and drying was over 80%, showing that loss during a large scale production will not cause any problem.
Comparison of efficacy between three WWEs using MI model
Efficacy of WWE of three varieties in reducing myocardial injury was investigated using a rat myocardial infarction (MI) model (Lim et al., 2016). WWE at various doses were administered with rodent food for 3 days (Fig. 2A), LAD was occluded for 30 min to make an ischemic condition, and then reperfused for 3 h to make a normoxic condition (Fig. 2A, 2B). To get area at risk (AAR) where blood was not supplied during occlusion, Evans blue dye was infused after LAD was occluded again (Fig. 2B). To get infarct area (IA) where white region represents MI, TTC staining was performed after the harvested rat hearts were excised into 4 pieces (Fig. 2B). Infarct size (IS), IA/AAR ratio, was adopted to reflect variations in AAR (Fig. 2C). In addition, the left ventricular area (LVA) was also assessed to get risk size (RS), AAR/LVA. Supplementation of Anbaek WWE at 63 and 250 mg/kg per day significantly reduced IS compared with the control group (26.7 ± 6.93 and 35.5 ± 5.56% vs 48.4 ± 2.1%, respectively, p < 0.05) (Fig. 3). In contrast, supplementation of Anzunbaengi WWE at 63 or 250 mg/kg per day did not reduce IS significantly compared with the control group (47.5 ± 4.1 and 41.9 ± 3.0% vs 48.4 ± 2.1%, respectively, p > 0.05). In addition, supplementation of Goso WWE at 63 mg/kg per day did not reduce IS significantly compared with the control group (48.9 ± 2.4% vs 48.4 ± 2.1%, p > 0.05). The RS of WWE-treated group was not significantly different from that of the control group (p > 0.05), which indicates that the surgical procedures to generate ischemia/reperfusion (I/R) injury was reliable (Fig. 3). These results showed that Anbaek WWE reduced myocardial injury at the lowest dose. Therefore, we chose Anbaek variety as the final candidate for further study. In our previous study, we showed that Kumkang WWE did not reduce IS at 1 g/kg per day in the same rat model as used in this study (Lim et al., 2016). Therefore, we concluded that Anbaek WWE showed efficacy at a lower dose than Kumkang WWE as well as Goso WWE and Anzunbaengi WWE.
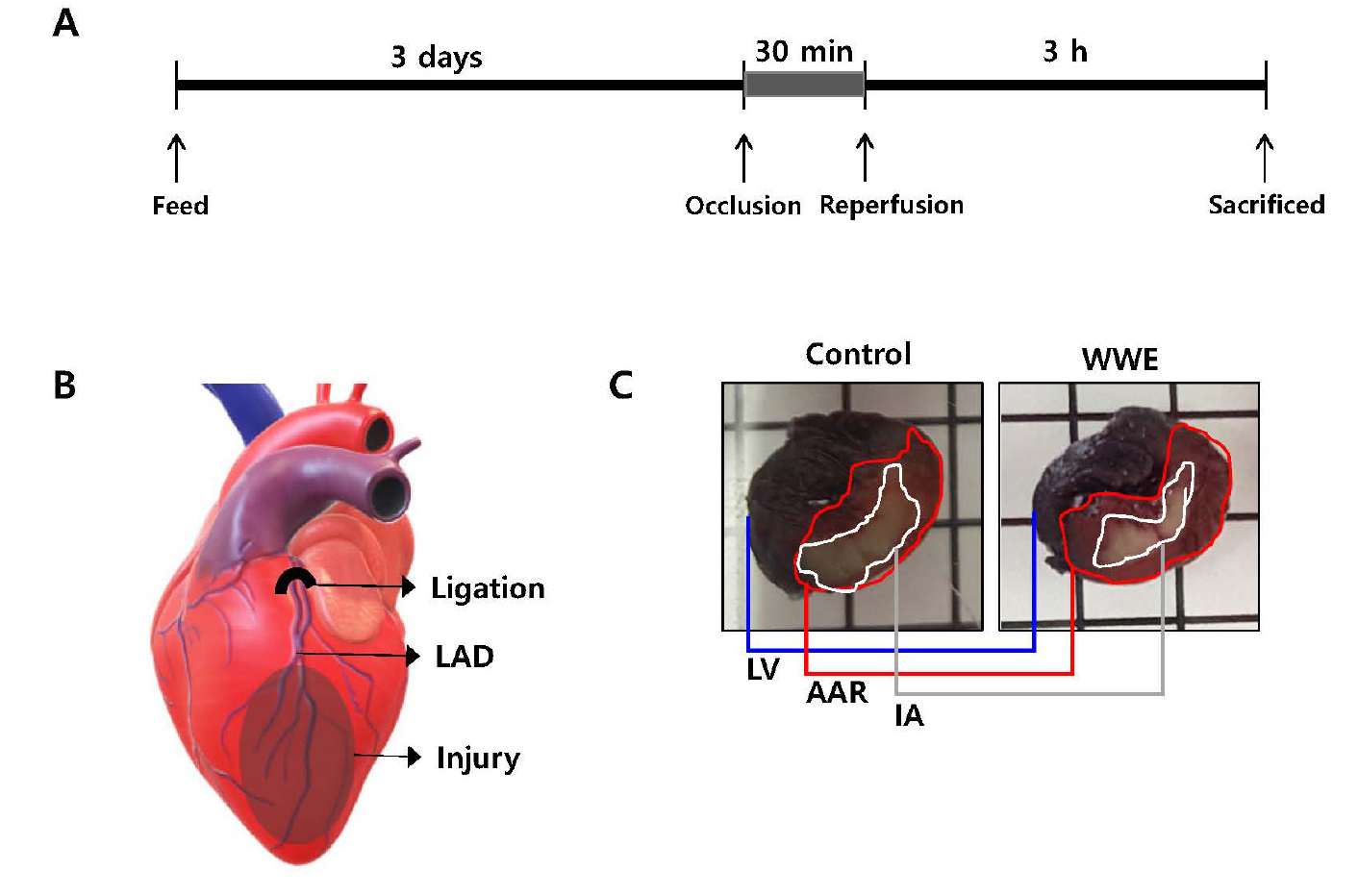
Fig. 2.
The experimental scheme in myocardial infarction experiments.
A and B: Rats were fed whole-wheat extract (WWE) at various dosages (0, 31, 63, 125, and 250 mg/kg) for 3 days, followed by occlusion through ligation of the left anterior descending artery (LAD) (30 min) and subsequent reperfusion through release of the ligation (3 h). The LAD was religated followed by an infusion of Evans blue dye. Subsequently, the rats were sacrificed, and their hearts were harvested. C: The harvested heart was cut into four slices, including the left ventricle (LV), and were stained with 2,3,5-triphenyltetrazolium chloride (TTC). The area at risk (AAR) and infarct area (IA) were determined as the area without Evans blue dye infiltration, and as the area not stained with TTC in AAR, respectively.
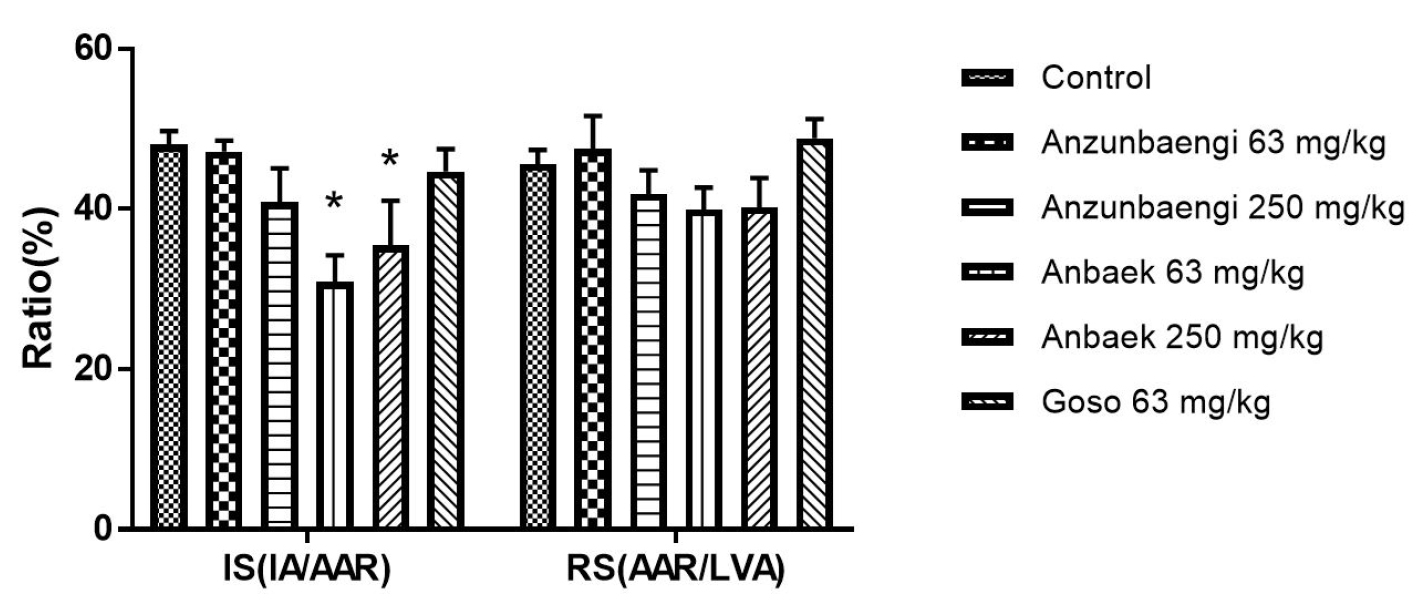
Fig. 3.
Effect of whole-wheat extract (WWE) of three Korean wheat varieties on infarct size in rats.
The WWE of three Korean wheat varieties (Anzunbaengi, Anbaek, and Goso) was supplemented with rodent food. Infarct size (IS) and risk size (RS) were assessed. IS, the ratio of the infarct area (IA) to the area at risk (AAR), and RS, the ratio of AAR to the left ventricular area (LVA), are presented as ratios (%). Data represent mean ± standard error of the mean (SEM) (n=8 for the control group; n=5 for the Anzunbaengi, Anbaek, and Goso groups). *p < 0.05, compared with the control group.
Efficacy of Anbaek WWE at various doses in MI model
To investigate the efficacy for Anbaek WWE at wider range of doses, we added 31 and 125 mg/kg per day in addition to 63 and 250 mg/kg per day as already shown in Fig. 3 (Fig. 4). Supplementation of Anbaek WWE at 31 and 125 mg/kg per day also significantly reduced IS compared with the control group (34.1 ± 5.2 and 34.7 ± 4.0% vs 48.1 ± 1.7%, respectively, p < 0.05). Thus, Anbaek WWE reduced myocardial injury in the all the ranges tested (31 – 250 mg/kg per day). To convert doses for rats into those for humans, we adopted a conversion factor recommended by Food & Drug Administration (FDA), in which 100 mg/kg per day for rats is equivalent to approximately 1 g/day for 60 kg persons (Nair and Jacob, 2016). Therefore, 31 mg/kg per day, the lowest dose showing efficacy for rats in this study, is equivalent to approximately 300 mg/kg per day for 60 kg persons. Considering variation of body weight in general population, we recommend 1 g/day Anbaek WWE for a person to prevent MI.
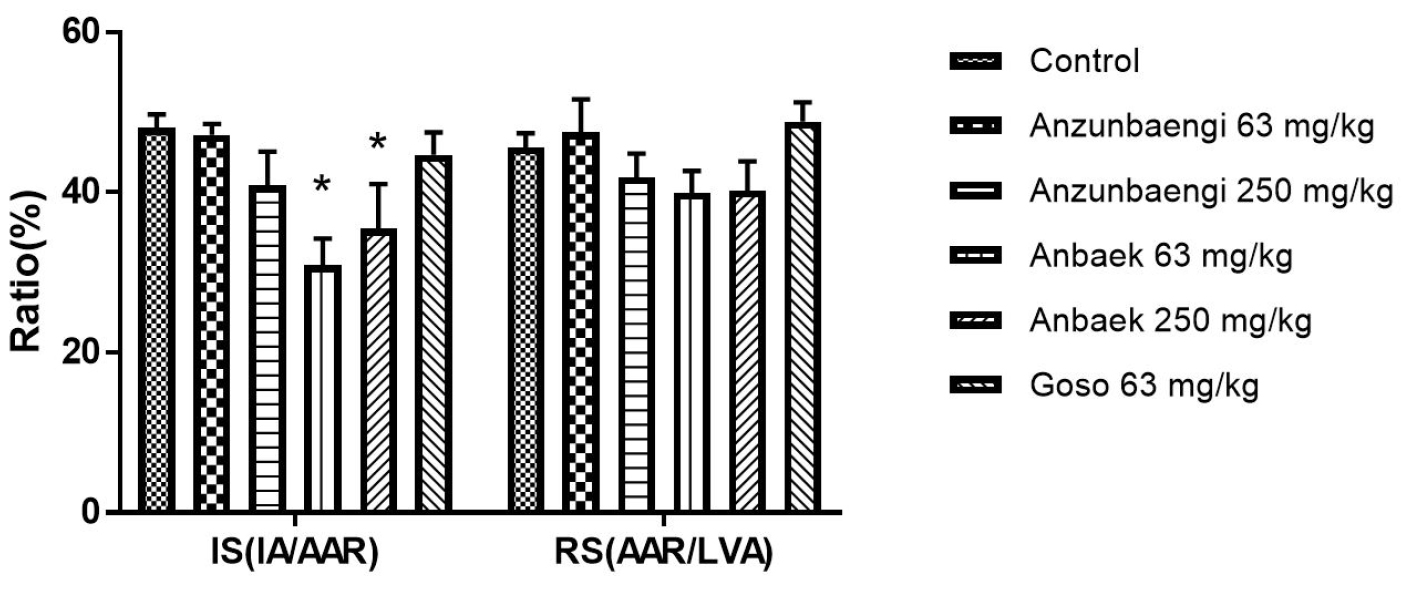
Fig. 4.
Effect of whole-wheat extract (WWE) of Anbaek wheat variety at various doses on infarct size in rats.
Anbaek WWE was supplemented with rodent food. Infarct size (IS) and risk size (RS) were assessed. IS, the ratio of IA to AAR, and RS, the ratio of AAR to the left ventricular area (LVA), are presented as ratios (%). Data represent mean ± SEM (n=8 for the control group; n=5 for the Anzunbaengi, Anbaek, and Goso groups). *p < 0.05 and **p < 0.01, compared with the control group, respectively.
Correlation between AX content and efficacy in MI model
To correlate between AX content in various Korean varieties and efficacy in the MI model, we measured contents of WE-AX and WU-AX in wheat flour of the varieties (Table 1). WE-AX in wheat flour of Anbaek, Goso and Kumkang were 15.0 ± 2.3, 8.0 ± 2.8, and 9.4 ± 0.2 mg/g, respectively (Table 1), resulting in the ratios between each variety as 1:0.53:0.63, respectively. Therefore, Anbaek wheat flour contains approximately twice as much as WE-AX than Goso and Kumkang wheat flour. On the other hand, WU-AX in wheat flour of Anbaek, Goso and Kumkang were 17.5 ± 0.2, 11.9 ± 0.8, and 14.3 ± 0.5 mg/g, respectively (Table 1), resulting in the ratios between each variety as 1:0.68:0.82, respectively. WU-AX content of Kumkang is close to that of Anbaek. Therefore, WE-AX content in each variety correlates more closely to the efficacy in the MI model than WU-AX. In other words, Anbaek containing the highest WE-AX content showed efficacy at the lowest dose. The results are consistent with those from previous studies showing that WE-AX should be better than WU-AX in producing arabinose and xylose, active monosaccharides (Lim et al., 2016), from hydrolysis of AX by acid in the stomach (Zhang et al., 2003) and by microorganisms inhabiting the large intestine (Koropatkin et al., 2012). From perspective of WE-AX content, Shinmichal 1 (18.1 ± 0.4 mg/g), Hanbaek (15.6 ± 0.9 mg/g) and Seodun (15.2 ± 0.5 mg/g) contain as much as or more WE-AX than Anbaek. Therefore, it is recommended to test whether these varieties are also as effective as Anbaek in reducing myocardial injury at a low dose.
Table 1.
Water-extractable arabinoxylan and water-unextractable arabinoxylan contents in Korean wheat varieties
| Cultivars |
WE-AX1) (mg/g)3) |
WU-AX2) (mg/kg) |
Cultivars |
WE-AX (mg/kg) |
WU-AX (mg/kg) |
| Alchan | 12.93 ± 0.244) | 19.04 ± 0.02 | Jojoong | 9.50 ± 1.25 | 14.24 ± 0.85 |
| Anbeak | 14.96 ± 2.28 | 17.48 ± 0.16 | Jokyung | 9.75 ± 1.21 | 11.98 ± 0.67 |
| Baekchal | 8.05 ± 1.16 | 11.61 ± 1.63 | Jonong | 7.84 ± 1.34 | 10.47 ± 0.24 |
| Baekkang | 8.57 ± 0.88 | 12.51 ± 1.63 | Jopoom | 9.92 ± 1.17 | 12.13 ± 0.70 |
| Baekjoong | 12.59 ± 1.15 | 13.33 ± 0.41 | Keumkang | 9.36 ± 0.18 | 14.32 ± 0.53 |
| Cheongkye | 12.78 ± 1.13 | 14.48 ± 0.64 | Milsung | 8.20 ± 2.41 | 12.38 ± 0.76 |
| Dabun | 8.20 ± 0.80 | 13.45 ± 0.13 | Namhae | 9.36 ± 0.65 | 14.14 ± 0.77 |
| Dahong | 11.00 ± 1.68 | 0.18 ± 0.00 | Ol | 12.84 ± 0.66 | 13.56 ± 0.91 |
| Dajoong | 7.98 ± 2.77 | 11.90 ± 0.84 | Olgeuru | 13.86 ± 0.52 | 14.19 ± 0.21 |
| Eunpa | 11.60 ± 1.85 | 18.24 ± 1.12 | Saekungkang | 7.60 ± 1.09 | 14.10 ± 0.56 |
| Geuru | 12.33 ± 0.72 | 17.69 ± 1.38 | Saeol | 10.49 ± 1.03 | 15.48 ± 0.39 |
| Gobun | 12.20 ± 0.20 | 19.28 ± 0.37 | Seodun | 15.20 ± 0.46 | 20.01 ± 0.53 |
| Goso | 7.98 ± 2.77 | 11.90 ± 0.84 | Shinmichal | 8.20 ± 2.41 | 18.13 ± 1.14 |
| Hanbaek | 15.57 ± 0.88 | 18.24 ± 1.07 | Shinmichal1 | 18.09 ± 0.36 | 18.20 ± 0.56 |
| Hojoong | 12.59 ± 1.15 | 13.19 ± 0.75 | Sooan | 8.60 ± 1.09 | 13.3 ± 0.74 |
| Jeokjoong | 12.05 ± 1.16 | 12.72 ± 0.83 | Sookang | 9.50 ± 1.25 | 12.59 ± 0.89 |
| Jinpoom | 12.43 ± 1.91 | 20.71 ± 1.27 | Taejoong | 10.69 ± 1.15 | 15.00 ± 0.28 |
| Joa | 8.80 ± 1.22 | 10.13 ± 1.78 | Tapdong | 11.50 ± 1.08 | 14.49 ± 0.28 |
| Joeun | 12.67 ± 2.89 | 19.73 ± 0.85 | Uri | 9.14 ± 0.76 | 14.68 ± 0.43 |
| Johan | 12.57 ± 0.88 | 18.55 ± 0.69 | Yunbaek | 11.41 ± 1.20 | 13.18 ± 0.88 |
Efficacy and underlying mechanisms of Anbaek WWE in AD model
As Anbaek WWE reduced myocardial injury at the lowest dose, we tested whether Anbaek WWE could also improve memory using Y-maze and passive avoidance test in a mouse model of memory impairment induced by scopolamine. In the experiments, the WWE was orally administered (p.o.), scopolamine was intraperitoneally injected (i.p.) after 30 min of the WWE administration, and memory tests were performed another 30 min after the scopolamine injection (Fig. 5A). Scopolamine acts as an antagonist against muscarinic acetylcholine receptor, leading to amnesia (Hasselmann, 2014; Lakstygal et al., 2019), and amnesia induced by scopolamine in humans mimics closely the cognitive deficits associated with AD (Ebert and Kirch, 1998). As scopolamine has been used to identify drugs in animal studies which enhance cognition at synapses, such as donepezil, we used donepezil as a positive control (Buccafusco, 2009).
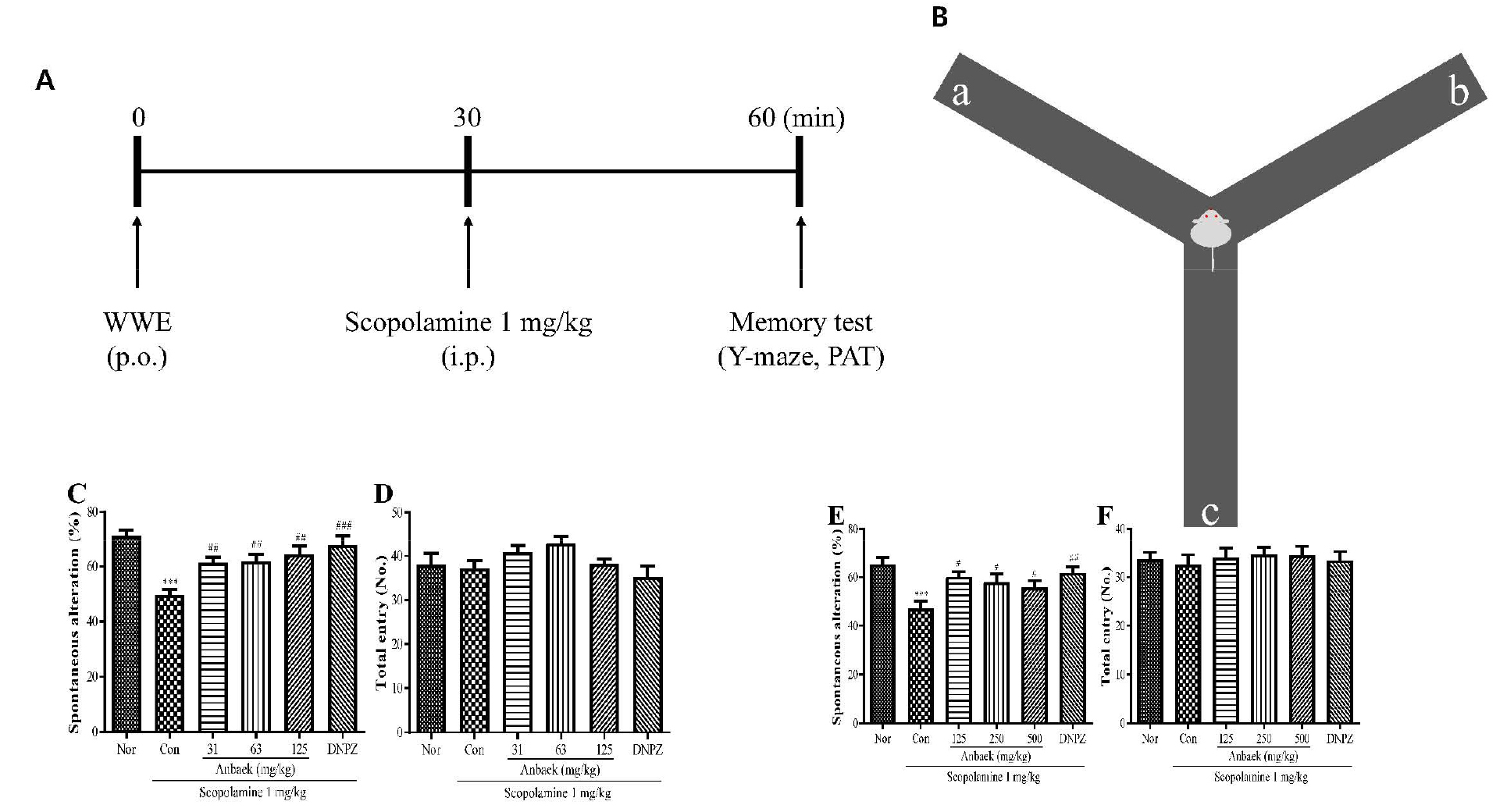
Fig. 5.
Effect of whole-wheat extract (WWE) of Anbaek wheat variety on memory improvement in mice (as assessed by the Y-maze test).
A: WWE was orally administered (p.o.). Scopolamine was intraperitoneally injected (i.p.) 30 min after the WWE administration. Subsequently, memory tests [Y-maze and passive avoidance test (PAT)] were performed another 30 min after the scopolamine injection. B: The Y-maze apparatus consists of three arms (a, b, and c) positioned at a 120° angle from each other. The Y-maze test measures the preference of mice to investigate a new arm of the maze rather than returning to the one that was previously visited and is presented as spontaneous alteration (%). C and D: WWE of Anbaek variety at lower doses (31, 63, and 125 mg/kg) and donepezil hydrochloride (DNPZ) at 5 mg/kg were orally administered. The spontaneous alteration (%) (C), and the number of total arm entries (D) were assessed. E and F: WWE of Anbaek variety at higher doses (125, 250, and 500 mg/kg) and DNPZ at 5 mg/kg dose were orally administered. The spontaneous alteration (%) (E), and the number of total arm entries (F) were assessed. In all these experiments, saline and scopolamine (1 mg/kg) were intraperitoneally injected for the normal group (Nor) and the control group (Con), respectively. Data represent mean ± SEM (n=9). ***p < 0.001, compared with the normal group. #p < 0.05, ##p < 0.01, and ###p < 0.001, compared with the control group.
Y-maze test was performed to assess whether Anbaek WWE improved memory impairment induced by scopolamine. Y-maze test that assesses reference memory and spatial working memory utilizes rodent’s inclination preferring to investigate a new arm of the maze rather than returning to one that was previously visited (in other words, spontaneous alternation) (Fig. 5B) (Webster et al., 2014). We first performed the Y-maze test at lower WWE doses (31, 63, and 125 mg/kg). Spontaneous alternation induced by scopolamine injection in the control group (Con) was significantly decreased compared with the normal group (Nor) (49.6 ± 2.1% vs 71.6 ± 1.9%) (p < 0.001), indicating that scopolamine impairs memory as expected (Fig. 5C). In contrast, spontaneous alternation in the donepezil-treated group (DNPZ) was significantly increased compared with the control group (67.7 ± 3.5% vs 49.6 ± 2.1%) (p < 0.001), indicating that donepezil reversed memory impairment induced by scopolamine. Spontaneous alternation in the WWE-treated groups at lower doses (31, 63, and 125 mg/kg) was significantly increased compared with the control group (61.3 ± 2.0%, 61.9 ± 2.4%, 64.4 ± 3.0%, respectively vs 49.6 ± 2.1%) (p < 0.01), and memory improvement tended to increase as doses increased. On the other hand, the number of total arm entry did not change significantly among each experimental group (Fig. 5D), indicating that scopolamine, WWE, and donepezil did not affect locomotor activity. Therefore, alternations observed in the experiments were not caused by changes in locomotor activity, but by changes in memory (Fig. 5C, 5D). We then performed Y-maze test at higher WWE doses (125, 250, and 500 mg/kg). As was observed at lower WWE doses, spontaneous alternation in the control group (Con) was significantly decreased compared with the normal group (Nor) (47.0 ± 3.1% vs 65.2 ± 3.0%) (p < 0.001), and spontaneous alternation in the donepezil-treated group (DNPZ) was significantly increased compared with the control group (61.7 ± 2.5% vs 47.0 ± 3.1%) (p < 0.01) (Fig. 5E). Spontaneous alternation in the WWE-treated group at higher doses (125, 250, and 500 mg/kg) was significantly increased compared with the control group (60.2 ± 2.0%, 57.8 ± 3.5%, 55.8 ± 2.7%, respectively vs 47.0 ± 3.1%) (p < 0.05). However, memory improvement tended to decrease as doses increased. Again, the number of total arm entry did not change significantly among each experimental group (Fig. 5F). The results showed that the WWE intake improved memory impairment induced by scopolamine injection in a wide range of doses (31 – 500 mg/kg), but exhibited maximum efficacy at the dose of 125 mg/kg in the Y-maze test.
Next, passive avoidance test was performed to assess whether Anbaek WWE improved memory impairment induced by scopolamine. Passive avoidance test that assesses reference memory and working memory, especially associative learning/memory, utilizes rodent’s inclination preferring dark environment and avoiding a dark environment in which an aversive stimulus such as foot shock was previously delivered (in other words, association of dark environment with foot shock) (Fig. 6A) (Webster et al., 2014). Therefore, it will take longer time (step-through latency in retention trials) for a mouse to enter a dark compartment where the mouse experienced foot shock previously if the mouse maintains shock memory. In passive avoidance test, we first performed the test at lower WWE doses (31, 63, and 125 mg/kg). Step-trough latency in retention trials (STLRT) induced by scopolamine injection in the control group (Con) was significantly shortened compared with the normal group (Nor) (70.6 ± 5.9 sec vs 271.8 ± 18.5 sec) (p < 0.001), indicating that scopolamine impairs memory as expected (Fig. 6B). In contrast, STLRT in the donepezil-treated group (DNPZ) was significantly lengthened compared with the control group (Con) (280.1 ± 15.2 sec vs 70.6 ± 5.9 sec) (p < 0.001), indicating that donepezil reversed memory impairment induced by scopolamine. STLRTs in the WWE-treated groups at lower doses (31, 63, and 125 mg/kg) were 90.1 ± 14.4 sec, 131.8 ± 20.2 sec, 232.9 ± 24.0 sec, respectively, showing that STLRTs were lengthened as doses increased. Among the lower doses, STLRTs at 63 and 125 mg/kg only were significantly lengthened compared with the control group (70.6 ± 5.9 sec) (p < 0.05, and p < 0.001, respectively), indicating that the WWE significantly reversed memory impairment induced by scopolamine at doses over 63 mg/kg. On the other hand, step-through latency in acquisition trial that represents time period staying in the illuminated compartment did not change significantly among each experimental group, indicating that experimental environment did not affect STLRT. We then performed passive avoidance test at higher WWE doses (125, 250, and 500 mg/kg). As was observed for lower WWE doses, STLRT in the control group (Con) was significantly shortened compared with the normal group (Nor) (45.9 ± 7.4 sec vs 257.3 ± 17.0 sec) (p < 0.001), and STLRT in the donepezil-treated group (DNPZ) was significantly lengthened compared with the control group (237.9 ± 22.0 sec vs 45.9 ± 7.4 sec) (p < 0.001). STLRTs in the WWE-treated groups at higher doses (125, 250, and 500 mg/kg) were 206.4 ± 27.4 sec, 130.6 ± 29.3 sec, and 81.0 ± 20.8 sec, respectively, showing that STLRT was shortened as doses increased (Fig. 6C). Among the higher doses, STLRTs at 125 and 250 mg/kg only were significantly lengthened compared with the control group (45.9 ± 7.4 sec) (p < 0.001, p < 0.05, respectively), indicating that the WWE significantly reversed memory impairment induced by scopolamine at doses lower than 250 mg/kg. Again, step-through latency in acquisition trial that represents time period staying in the illuminated compartment did not change significantly among each experimental group (Fig. 6C). The results showed that the WWE intake improved memory impairment induced by scopolamine injection in certain range of doses (63 – 250 mg/kg), but exhibited maximum efficacy at the dose of 125 mg/kg. Putting the results from Y-maze and passive avoidance test together, one time administration of Anbaek WWE significantly improved memory impaired by scopolamine injection at the doses of 63, 125, and 250 mg/kg, of which maximum efficacy was observed at the dose of 125 mg/kg. To convert doses for mice into those for humans, we again adopted a conversion factor recommended by FDA, in which 100 mg/kg per day dose for mice is equivalent to approximately 0.5 g/day for 60 kg persons (Nair and Jacob, 2016). Therefore, 63 mg/kg per day, the lowest dose showing efficacy for mice in this study, is equivalent to approximately 320 mg/kg per day for 60 kg persons. Considering variation of body weight in general population, we recommend 1 g/day Anbaek WWE to prevent AD.
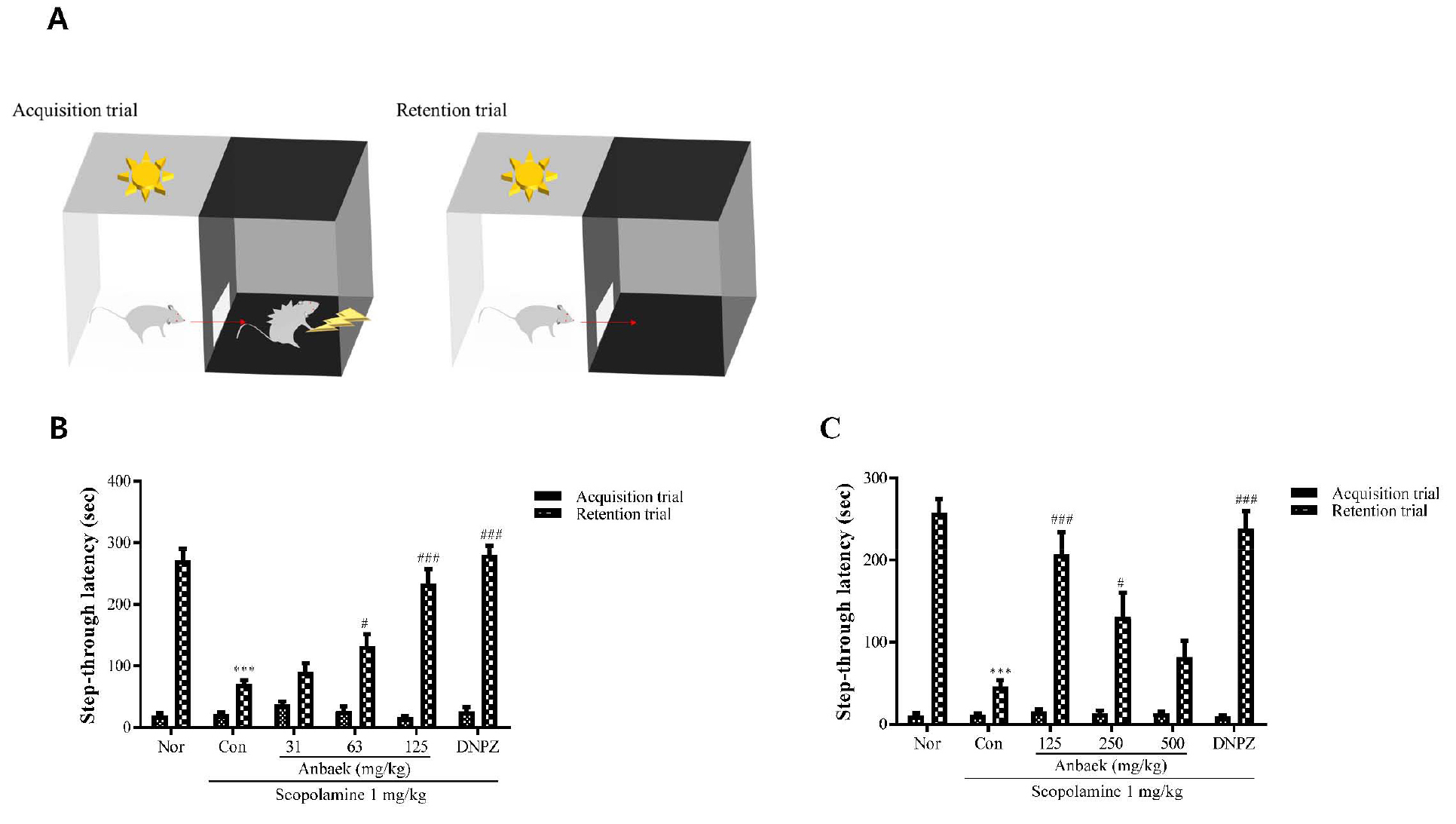
Fig. 6.
Effect of whole-wheat extract (WWE) of Anbaek wheat variety on memory improvement in mice (as assessed by the passive avoidance test).
A: The passive avoidance test apparatus consists of two compartments, illuminated and dark, which are separated by a guillotine door. When a mouse is placed in the illuminated compartment, the mouse moves into the dark compartment due to its preference for a dark environment. In the acquisition trial, a mouse was placed in the illuminated compartment. Step-through latency (sec), the time taken for the mouse to move into the dark compartment, was measured. A mild foot shock was then administered to the mouse. In the retention trial, the mouse was again placed in the illuminated compartment on the following day and step-through latency was measured again. B: WWE of Anbaek wheat variety at lower doses (31, 63, and 125 mg/kg) and donepezil hydrochloride (DNPZ) at 5 mg/kg dose were orally administered. The step-through latency in the acquisition and retention trials was measured. C: WWE of Anbaek wheat variety at higher doses (125, 250, and 500 mg/kg) and DNPZ at 5 mg/kg dose were orally administered. The step-through latency in the acquisition and retention trials was measured. In experiments described in (B) and (C), saline and scopolamine (1 mg/kg) were intraperitoneally injected for the normal group (Nor) and the control group (Con), respectively. Data represent mean ± SEM (n=9). ***p < 0.001, compared with the normal (Nor) group. #p < 0.05 and ###p < 0.001, compared with the control (Con) group.
To elucidate underlying mechanisms how Anbaek WWE improved memory impairment induced by scopolamine, we focused on synaptic proteins because scopolamine acts at synapses (Hasselmann, 2014; Lakstygal et al., 2019), and synaptic dysfunction is involved in the early stage of AD progression (Chen et al., 2019; Jackson et al., 2019; Pei et al., 2020). Thus, we tested whether several proteins involved in synaptic transmission were modulated by Anbaek WWE. We chose phosphorylated synapsin 1 (p-SYN1) as representing presynaptic proteins, and PSD95, neuroligin 3 (NL3) and GluN2B, a subunit of N-methyl-D-aspartate (NMDA) receptor (NMDAR), all as representing postsynaptic proteins (Torres et al., 2017). The modulation of these proteins can be summarized as follows; Synapsin 1 in dephosphorylated state (SYN1) tethers synaptic vesicles to the cytoskeleton, leading to inhibiting release of neurotransmitter from presynaptic neurons. Synapsin 1 in phosphorylated state (p-SYN1), however, mobilize synaptic vesicles, leading to allowing release of neurotransmitters such as glutamate (Jovanovic et al., 2000; Mirza et al., 2018). Glutamate released into synaptic clefts binds to glutamate receptors on the membranes of postsynaptic neurons such as NMDAR, leading to regulation of synaptic plasticity and memory formation (Torres et al., 2017). PSD95, a scaffolding protein located in the postsynaptic neurons, is involved in the stabilization, recruitment, and trafficking NMDAR to the postsynaptic membranes, leading to enhancing glutamatergic neurotransmission, and synaptic plasticity (Coley and Gao, 2018; Torres et al., 2017). Finally, NL3, an adhesion molecule located on the postsynaptic membranes, forms complex with neurexins located on the presynaptic membranes (Coley and Gao, 2018; Sudof, 2008; Torres et al., 2017). Formation of the complex promotes synapse formation and synaptic function, and mutations in NL3 disrupting complex formation cause cognitive impairment (Bassani et al., 2013). For the assessment, hippocampi were harvested from the brains of mice receiving 125 mg/kg of Anbaek WWE, from which proteins were extracted. When levels of p-SYN1, PSD95, NL3, and GluN2B were assessed via western blotting, administration of Anbaek WWE significantly upregulated p-SYN1, PSD95 and GluN2B compared with the control group, respectively (Fig. 7A, 7B). In addition, administration of Anbaek tended to upregulate NL3 compared with the control group (Fig. 7A, 7B). Based on these results, we propose an underlying mechanism in which Anbaek WWE improves memory impairment induced by scopolamine (Fig. 7C). WWE enhanced glutamate release from presynaptic terminal by mobilizing presynaptic vesicles through SYN1 phosphorylation. Neurotransmission via glutamate binding to NMDAR was increased by upregulation of NMDAR and PSD95. In addition, synapse formation was promoted by upregulation of NL3 expression. Putting these results together, Anbaek WWE improved memory by enhancing glutamatergic neurotransmission at the excitatory synapses in the hippocampus.
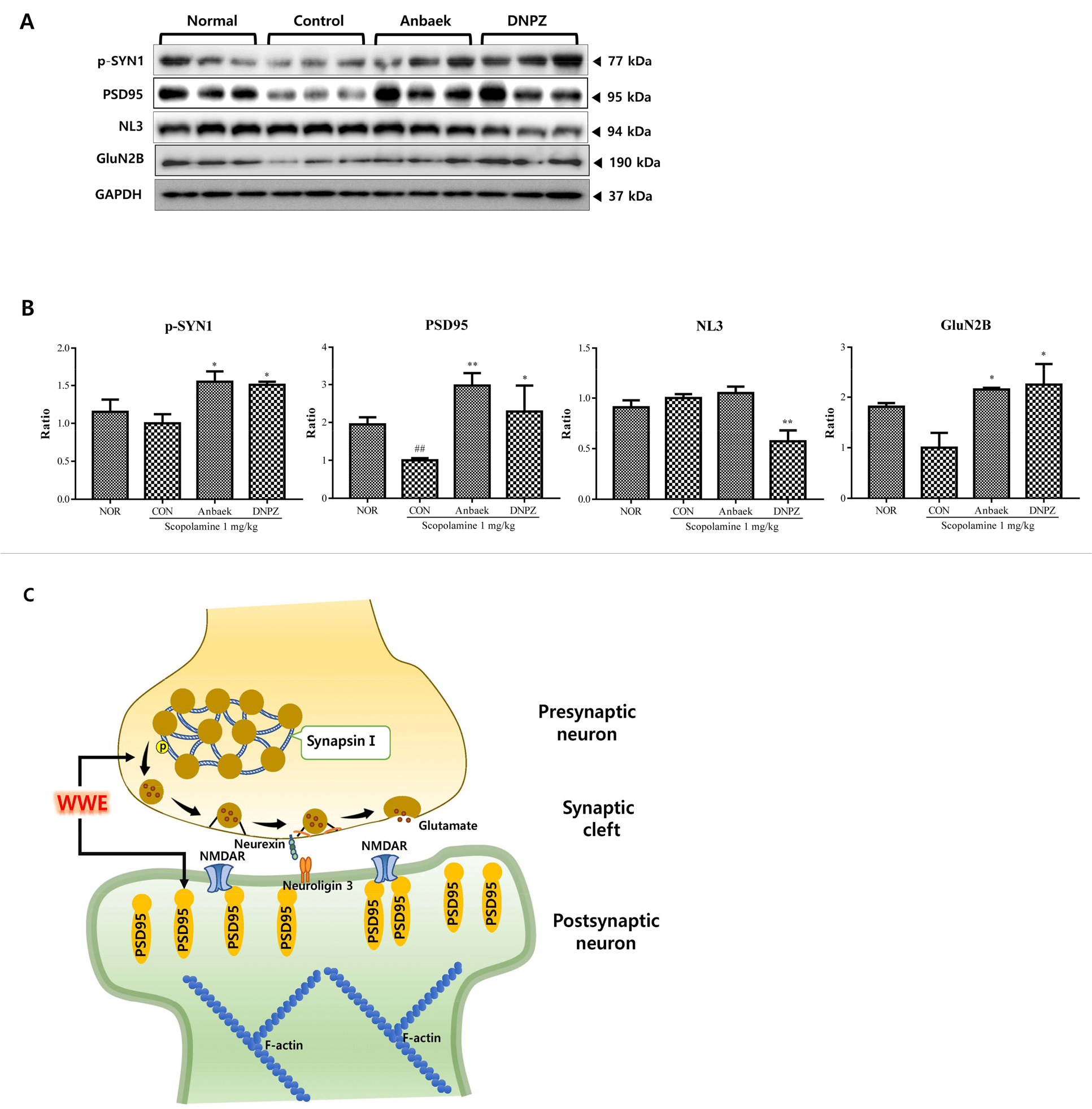
Fig. 7.
Effect of whole-wheat extract (WWE) of Anbaek wheat variety on the expression of p-SYN1, PSD95, NL3, and GluN2B in the hippocampus after the Y-maze test.
Western blots of p-synapsin 1 (p-SYN1), postsynaptic density 95 (PSD95), neuroligin 3 (NL3), and NMDA receptor 2B (GluN2B) are presented. A: Expression levels of the proteins in the hippocampus determined by western blotting for the normal (Nor), control (Con), Anbaek WWE-treated (125 mg/kg), and donepezil-treated (DNPZ) (5 mg/kg) groups are presented. GAPDH was used as a loading control. B: Quantitative analysis of p-SYN1, PSD95, NL3, and GluN2B are presented. The ratios were calculated by setting the control group (Con) value (0 mg/kg WWE) at 1. Data represent mean ± SEM (n= 3). **p < 0.01, compared with the normal (Nor) group. #p < 0.05 and ##p < 0.01, compared with the control (Con) group. C: A hypothesized underlying mechanism for memory improvement in the hippocampus by Anbaek WWE is presented. WWE improves neurotransmission through a synapse comprising a presynaptic neuron, synaptic cleft, and a postsynaptic neuron. WWE enhances glutamate release from the presynaptic terminal by mobilizing presynaptic vesicles through SYN1 phosphorylation. Neurotransmission via glutamate binding to NMDA receptor is increased by the upregulation of NMDA receptor and PSD95. In addition, synapse formation was promoted by the upregulation of NL3 expression.
Putting the results from MI and AD models together, we recommend intake of 1 g/day Anbaek WWE to prevent MI and AD. However, unlike results from MI experiments, administration of WWE at higher doses (above 250 mg/kg) in AD model started to inhibit memory improvement. These phenomena imply that some ingredients in WWE may interfere with memory formation and storage at higher doses. One ingredient that can be considered is gluten, the major protein in the wheat grains. Gluten supplementation to rats one time reduced memory after memory was assessed after 30-60 min of gluten administration (Du et al., 2018). In addition, gluten intake makes individuals sensitive to gluten concentrate difficult and leads to brain fog characterized by forgetfulness, confusion, and lack of mental clarity (George et al., 2021; Yelland, 2017). Therefore, it is necessary to investigate whether gluten administration would cause memory impairment under our experimental conditions. As such, we can expand effective dose ranges of Anbaek WWE intake which show maximum efficacy.




