Introduction
Materials and Methods
Samples
Physicochemical analysis of deep seawater salt
Assay for in vitro inflammatory effect
Statistical analysis
Results
Chemical composition
Content of mineral elements by ICP-OES
Cytotoxicity of DS
Effect of DS on NO production
Effect of DS on LPS-induced iNOS and COX-2 protein expression
Effect of DS on immune-promoting cytokines
Discussion
Thus, it can be concluded by the followings from these studies
Summary and Future Implications
Introduction
Deep seawater exists at depths of over 200 m under the sea. It contains a large amount of mineral salts, of which cleanness is maintained. As no sunlight reaches it, photosynthesis does not take place there and contains no organic matter. In addition, the temperature is maintained at a stable low level throughout the year, so it hardly mixes with the sea water on the surface (Hataguchi et al., 2005).
Salt is used in food as a flavor enhancer, bringing out the natural taste of foods. It is also used to regulate fermentation, attain the proper texture, and for preservation in foods. Recently, a growing number of people are aware of the limitations and health risks of taking an improper amount of salts. We are becoming aware of the importance of the function of quality salt intake to avoid health risks such as hypertension, cancer, heart attack, and many senile diseases. Salt is an essential nutrient, and regular intake is required to maintain many essential body functions.
Salt is one of the absolutely necessary nutrients in human life and takes part in many physiological processes of the human body. The essential components of salt are sodium and chlorine, which are necessary for the human body. Sodium chloride ensures the balance of acid and alkali, electrolytic balance in the human body as well. The human body produces many acidic materials, and it is the sodium cushioning effect that maintains the body’s weak alkalinity (Livingston, 2005).
The kind of salt can be classified according to the components. In general, mineral salt contains sodium, chloride, potassium, calcium, phosphorus, and magnesium. Highly refined salt used at home is extracted pure sodium chloride, often reaching a purity of 99.9%, with close to zero other compounds. Often, toxic heavy metals are detected in salt from polluted seawater, particularly in industrial areas. However, there is no room for contamination in deep seawater salt. Therefore, various salt products can be developed, such as high calcium salt, low sodium salt, and high magnesium salt. Inflammation is the body’s immediate response to tissue and cell damage caused by pathogens or other harmful stimuli. Acute inflammation is a short-term response that typically leads to healing. It involves leukocytes infiltrating the damaged area, removing the stimulus, and repairing the tissue. On the other hand, chronic inflammation is a prolonged, deregulated, and maladaptive response characterized by ongoing inflammation and tissue destruction. Persistent inflammation is associated with numerous chronic human conditions and diseases, including allergy, atherosclerosis, cancer, arthritis, and autoimmune diseases (Weissler et al., 2008).
Studies on the anti-inflammatory property of deep seawater salt and its mechanism of action in the RAW 264.7 macrophage cell line are limited. However, the use of RAW 264.7 cells is considered suitable for studying the inflammatory response in cultured cells. These cells release nitric oxide (NO) and cytokines such as TNF-α, IL-1β, and IL-6 when stimulated with Lipopolysaccharide (LPS). Co-induction or co-regulation of COX-2 and iNOS has been demonstrated in various cell culture studies and animal inflammatory models, including in RAW 264.7 cells (Vane et al., 1994).
In this study, the focus was to understand the physicochemical properties of deep seawater salt. Subsequently, the study aimed to investigate the anti-inflammatory properties, immune-enhancing effects of the salt.
Materials and Methods
Samples
Deep seawater salt was obtained from Gangwon Deep Sea Water Co., (Gosung, Gangwon State, Korea) and the solar dried salt (Shinan, Jeonnam Province) was purchased from the local market. Each salt sample used was dried for 5 h at 80°C.
Physicochemical analysis of deep seawater salt
Analysis of quality characteristics
Analysis of the components of deep seawater salt was carried at Gangneung Wonju National University, measuring sodium chloride, insoluble component, a sulfate ion, moisture content and heavy metal content according to the Food Code experimental methods.
Analysis of mineral content (ICP-OES)
Deep seawater and solar dried salts of mineral contents were determined by ICP-OES (Inductively Coupled Plasma-Optical Emission Spectrometry; OPTIMA 7300 DV, Perkin-Elmer, Waltham, MA, USA). Samples were prepared following the instrument manuscript. Briefly, salts were diluted 1:100 with deionized water for the determination of Na, Ca, Mg, P, Fe, Mn and K. These data were obtained in triplicate (Table 1).
Table 1.
ICP/OES instrument1) setting
Assay for in vitro inflammatory effect
Reagents
The reagents were purchased from the Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco BRL, Gaithersburg, MD, USA). iNOS and COX-2 (BD Transduction Laboratories), β-actin (Santa Cruz Biotechnology Inc., CA, USA), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) and LPS were purchased from Sigma (St. Louis, MO, USA).
Cell culture and cell viability assay
The RAW 264.7 cell line was purchased from the American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 10% FBS, 1% penicillin (100 U/mL) - streptomycin (100 µg/mL). To examine the effect of salts and LPS on cell viability, Cells were plated in 24-well plates at 40,000 cells/well with DMEM containing 10% FBS. One day later, the monolayers were serum deprived in DMEM containing 1% FBS for 24 h. After serum deprivation, cells were treated with 0.1, 0.2 mg/mL of deep seawater and solar dried salts in the absence or presence of LPS (1 µg/mL). Viable cell numbers were estimated by the 3-(4, 5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay as described by Cho (Cho et al., 2003).
Nitric Oxide (NO) and cytokine assays
RAW 264.7 cells were plated at 4 × 104 cells/well in 24-well plates and then incubated with or without LPS (1 µg/mL) in the absence or presence of various concentrations of salts for 24 h. The 24-h conditioned media were collected for NO and cytokine assays. The NO concentrations were measured using the Griess reagent system (Promega, Madison, WI, USA) and the concentrations of tumor necrosis factor (TNF)-α and interleukin (IL)-6 were measured using ELISA kits according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA).
Western blot analysis
iNOS and COX-2 expression were determined by Western blot analysis. Raw 264.7 cells were plated in 100-mm dishes at 1 × 105 cells/dish, serum deprived, and treated with 0.1, 0.2 mg/mL of Deep seawater and solar dried salts in the absence or presence of LPS. The cells were harvested in ice-cold lysis buffer consisting of 1% Triton X-100, 1% deoxycholate and 0.1% sodium dodecyl sulfate (SDS). The protein content of the cell lysates were then determined using BCA protein assay kit (Pierce, Rockford, IL, USA). Protein in each sample (15 µg total protein) were resolved by 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to the nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ, USA), blocked with 5% skim milk solution for 1 h, and the incubated with primary antibodies diluted (1:1000) for 3 h. The blots were then incubated with anti-mouse or rabbit HRP-conjugated secondary antibody for 1 h at room temperature. Each western blotting detection reagents and detected by chemiluminescence using Immobilon™ western chemiluminescent HRP Substrate (Millipore, Bedford, MA, USA). Densitometric analyses were conducted using an image J (Ver 1.42, NIH Image, developed and maintained by the National Institutes of Health, Bethesda, MD, USA) and the expression levels were normalized to β-actin, and the control levels were set at 100%.
Statistical analysis
Data from an individual experiment was expressed as the means ± standard error of means (SEM). The level of significance was set at p < 0.05. Statistical analysis was performed by GLM (general linear model) of SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) and Duncan’s multivariate test.
Results
Chemical composition
The chemical compositions of deep seawater salt are shown in Table 2.
Table 2.
Component criteria of the commercial salts and chemical characteristics of the DS (MFDS, 2002)
| Items | Deep seawater salt | Solar dried salt | Refined salt |
| (The present study) | (MFDS, 2002) | (MFDS , 2002) | |
| NaCl (%) | 93.8 | > 95 | > 88.0 |
| Chloride (%) | 58.8 | > 58 | > 54.0 |
| Moisture (%) | 0.3 | < 4.0 | < 9.0 |
| Insoluble solid (%) | 0.0 | < 0.02 | <0 .02 |
| SO4 ion (%) | 0.27 | < 0.4 | < 0.8 |
| Pb (mg/kg) | N.D1) | < 2.0 | < 2.0 |
| Cd (mg/kg) | N.D | < 0.5 | < 0.5 |
Deep seawater salt (DS) is shown the salinity of around 93.8%. Lee et al. (Lee and Kim, 2008) reported that the NaCl content of solar dried salt is 80-90%. NaCl content has a close relationship with the changes of seasons and moisture content, production time, storage location and storage time. In general, most of the NaCl on the market is refined salt. Ha and Park reported that high concentration of NaCl has not only comutagenicity but effect to promote peroxidation (Ju et al., 2017).
Moisture content of DS was 0.3%, markedly low compared with solar dried salt (8-12%) and refined salt (9%) (Min et al., 2020), these results similar to bamboo salt, is considered that closely related to removal of moisture by heating. pH is slightly acidic at 6.01 refined salt, solar dried salt showed a neutral 7.11.
Concerns of due to pollution of sea, Pb of one of the hazardous heavy metals is not easily excreted, an accumulation in the body, tissue dysfunction and acute and chronic diseases are known to induce (Jiang et al., 2021). Pb and Cd in insoluble component of salt have not been detected in the deep seawater salt. Deep seawater salt was not detected insoluble solid compared to 0.6-1.2% (Chien et al., 2019), of solar dried salt at west and south sea, 3% of bamboo salt. Deliquescence phenomenon occurs when insoluble content of these may not be sufficiently removed, chlorine, sulfate ions and moisture content is higher, reduced the quality of the salt (Lee and Kim, 2008).
Sulfate ion content of DS (0.27%), refined salt (0.8%) and solar dried salt (0.4%) satisfies edible salt composition the baseline of the Ministy of Food and Drug Safety (MFDS) Standards Codex (MFDS, 2002).
Content of mineral elements by ICP-OES
Mineral absorption is an essential process for the human beings, therefore mineral deficiency is very important. Calcium assists in the process of blood clotting and help to maintain the acid-alkaline balance in the blood. Phosphorus is an essential mineral that is usually found in nature which combined with oxygen. Health benefits of manganese ensure healthy bone structure, bone metabolism, helping in building essential enzymes for building bones (Shin et al., 2005).
To compare mineral elements of deep seawater salt and solar dried salt (Park et al., 2000), the content of Na, Ca, Mg, K, P, Fe and Mn were measured (Table 3). Mineral content such as Ca, P, K, Fe and Mn of deep seawater salt were higher than those of solar dried salt samples. Calcium content in deep seawater salt is 74.67 mg/L, which is 15.2% higher than that of solar dried salt (11.355 mg/L).
Table 3.
Mineral content obtained by ICP-OES analysis of DS (Concentration Unit: mg/L)
| Element | Deep seawater salt | Solar dried salt |
| Na | 1901.555 | 1885.288 |
| Ca | 74.670 | 11.355 |
| Mg | 16.667 | 63.415 |
| K | 34.874 | 19.106 |
| P | 0.240 | 0.151 |
| Fe | 0.202 | 0.093 |
| Mn | 0.103 | 0.022 |
According to the results of HANES I (Plam and Operation of the Health and Nutrition Examination Survey I) surveyed in the United States, result analyzed the correlation between hypertension occurs and nutrients of 17 kinds, Ca is shown a correlation between the most negative, Ca intake of hypertension patient was lower by 18% than healthy people (McCarron, 1983). P, Fe and Mn contents were also higher deep seawater salt that was in the trace. However, the Mg content of deep seawater salt was 3.8 times lower relative to that of solar dried salt. When the solar dried salt is stored for a long period of time, Mg influences the bitter taste of the salt. Generally, it is used by deleted for enhancing the taste of the food production (Sacco et al., 2006). Moon et al. (2005), reported that Ca, K and Mg in the spray dried salts are relatively higher than those in solar dried and bamboo salts, which is caused by their volatilization during spray and vaporization of the concentrated seawater.
Cytotoxicity of DS
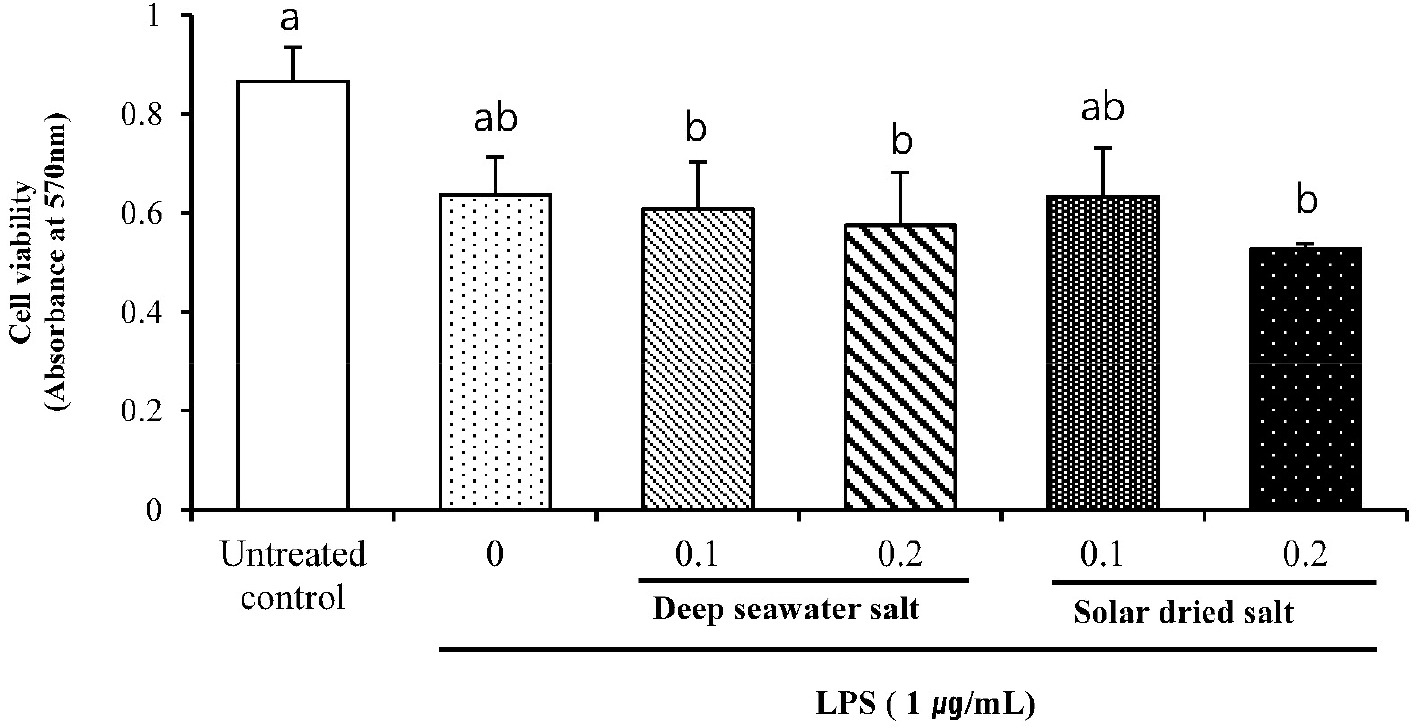
Macrophages play crucial roles in the initiation, maintenance, and resolution of inflammation (Kang et al., 2021). The cytotoxic effects of DS were evaluated in the presence and absence of LPS using the MTT assay.
Because cell viability was not significantly affected by DS up to 1.2 mg/mL (data not shown), chose concentration of 0.1, 0.2 mg/mL salts for the subsequent experiments. Fig. 1. showed that samples at 0.1 mg/mL concentration did not show any cytotoxicity to target cells, RAW 264.7 cell line.
Effect of DS on NO production
Nitric oxide is a highly reactive free radical and essential for host-immune responses to pathogens and NO has been recognized as a key player for the regulation of other physiological functions, such as vasodilation and neuronal activity. Especially, sustained expression of NO has been known to give macrophage with cytotoxic or cytostatic against viruses, bacteria, fungi, protozoa, and tumor cells (Lee and Park, 2015).
NO expression in treated RAW 264.7 was shown in Fig. 2. LPS markedly increased NO compared to untreated control and NO production in DS LPS-treated RAW 264.7 was significantly increased comparing to that of SS (P < 0.05). DS and SS were significantly increased in a dose-dependent manner compared to untreated control (LPS -).
The increase of NO production after DS or SS treatment on RAW 264.7 macrophage cell line is thought to be caused by immune response might be caused by immune promoting activity. There was no significant difference between DS and SS of 0.1 mg/mL. Thus, we focused more on DS of 0.2 rather than DS of 0.1 because 1 day incubation is more economical so that it has more benefits for application.
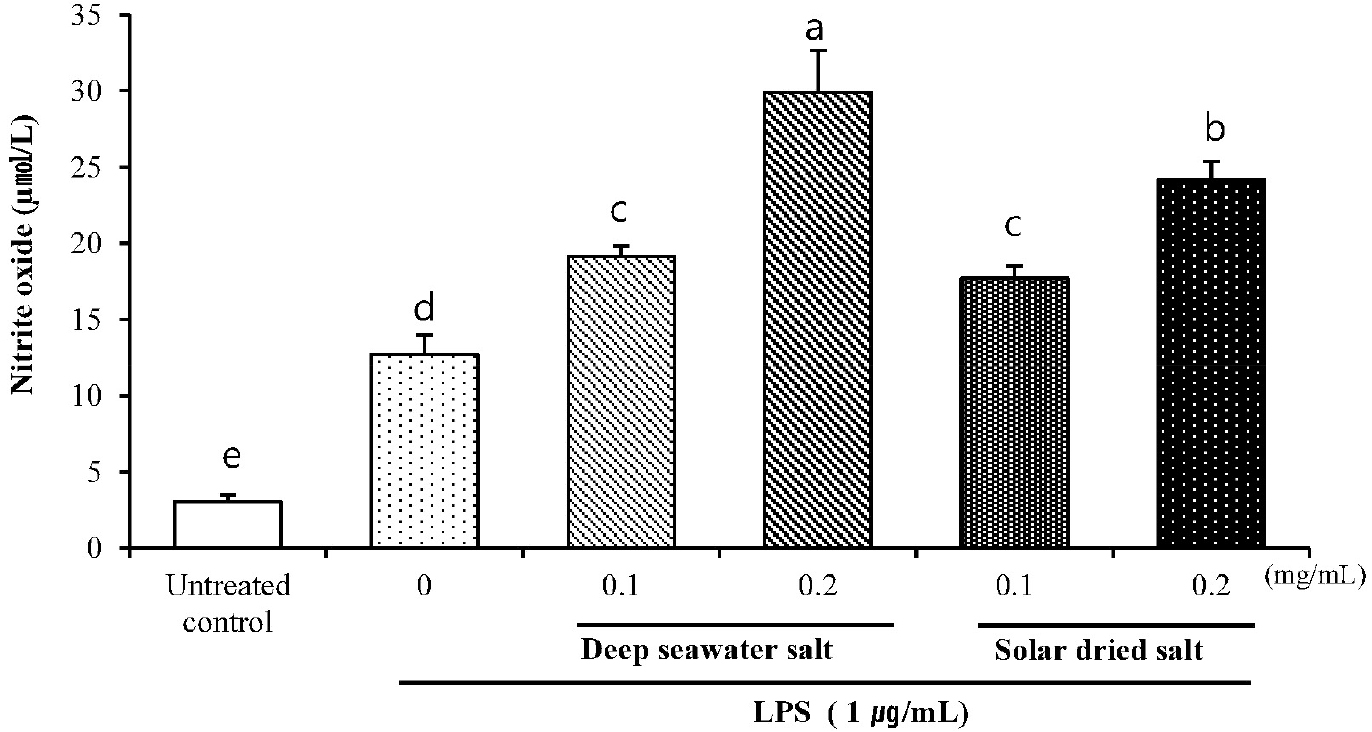
Fig. 2.
Effect of DS on NO production in LPS-induced RAW 264.7. Cells were pretreated with 0.1 and 0.2 (mg/mL) of DS and SS for 18 h and exposed to LPS (1 µg/mL) for 24 h. The nitrate production was measured by the Griess reaction assay. Data are represented as means ± SD of three independent experiments.
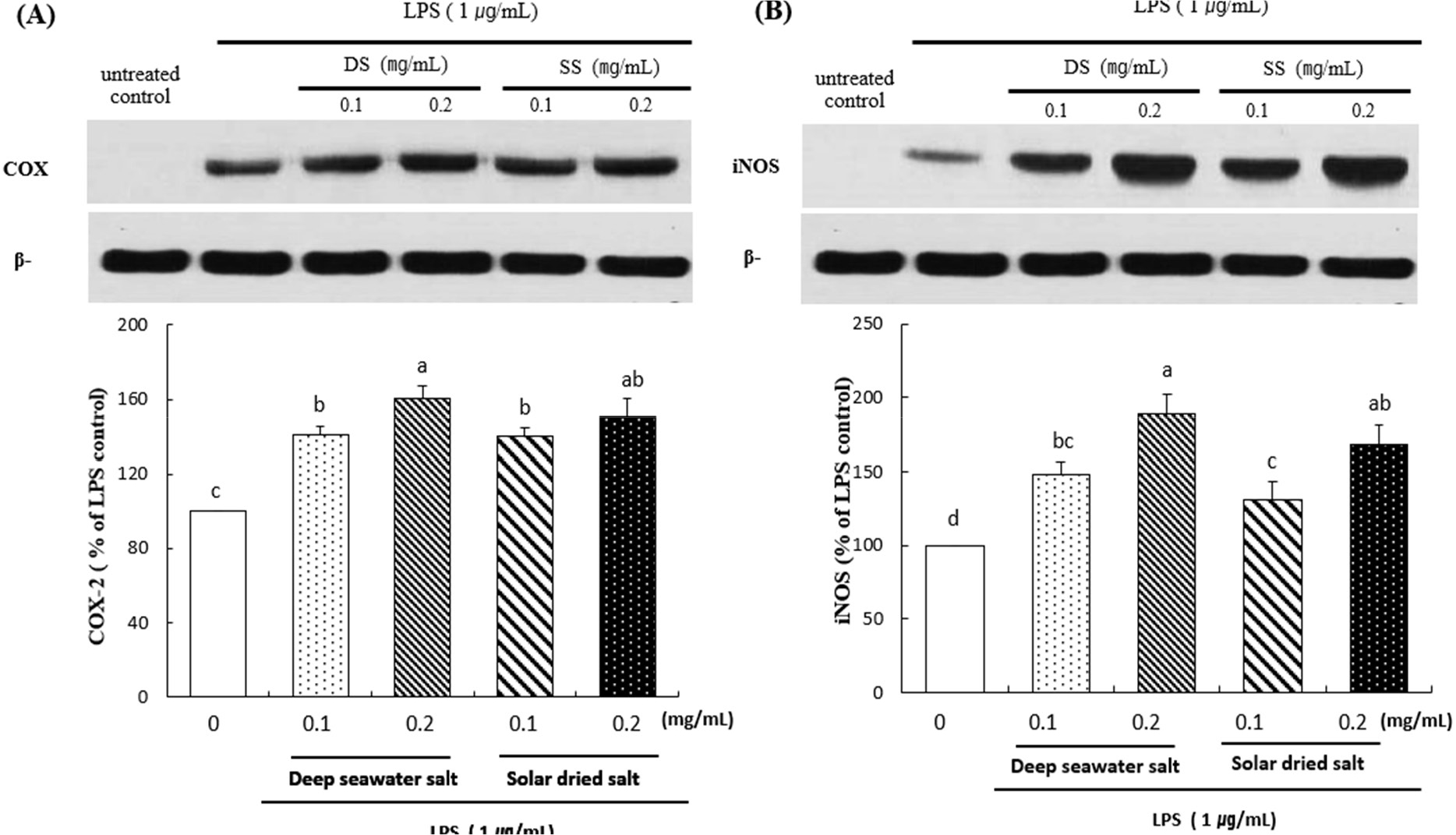
Effect of DS on LPS-induced iNOS and COX-2 protein expression
The results were shown that effect of deep seawater salt on the expression of iNOS and COX-2 (Fig. 3).
Western blot analysis revealed that DS was dose-dependently increased LPS-induced protein expression of iNOS and COX-2 protein levels. DS and SS treated group represents significant difference compared to untreated control (p < 0.05). DS of 0.1 (mg/mL) treated cells showed similar amounts of expression of iNOS compared to that of SS at the same concentration, but DS of 0.2 (mg/mL) was significantly increased comparing to that of SS of 0.2 (mg/mL) (P < 0.05). Overall, the results revealed that treatment with deep seawater salt showed the better anti-inflammation effect than solar dried salt.
Effect of DS on immune-promoting cytokines
TNF-α and IL-6 in treated RAW 264.7 were shown in Fig. 4 and 5. TNF-α was dramatically expressed in DS treated cells. DS and SS treated group represents significant difference of TNF-α compared to untreated control. DS of 0.1 (mg/mL) treated cells showed similar amounts of expression compared to that of SS at the same concentration, but DS of 0.2 (mg/mL) was significantly increased comparing to that of SS of 0.2 mg/mL (P < 0.05).
DS and SS treated group represents significant difference of IL-6 in a dose-dependent manner compared to untreated control (P < 0.05). Even though there was no significant difference in LPS treated group, it also showed a slight increase in DS and SS treated cells.
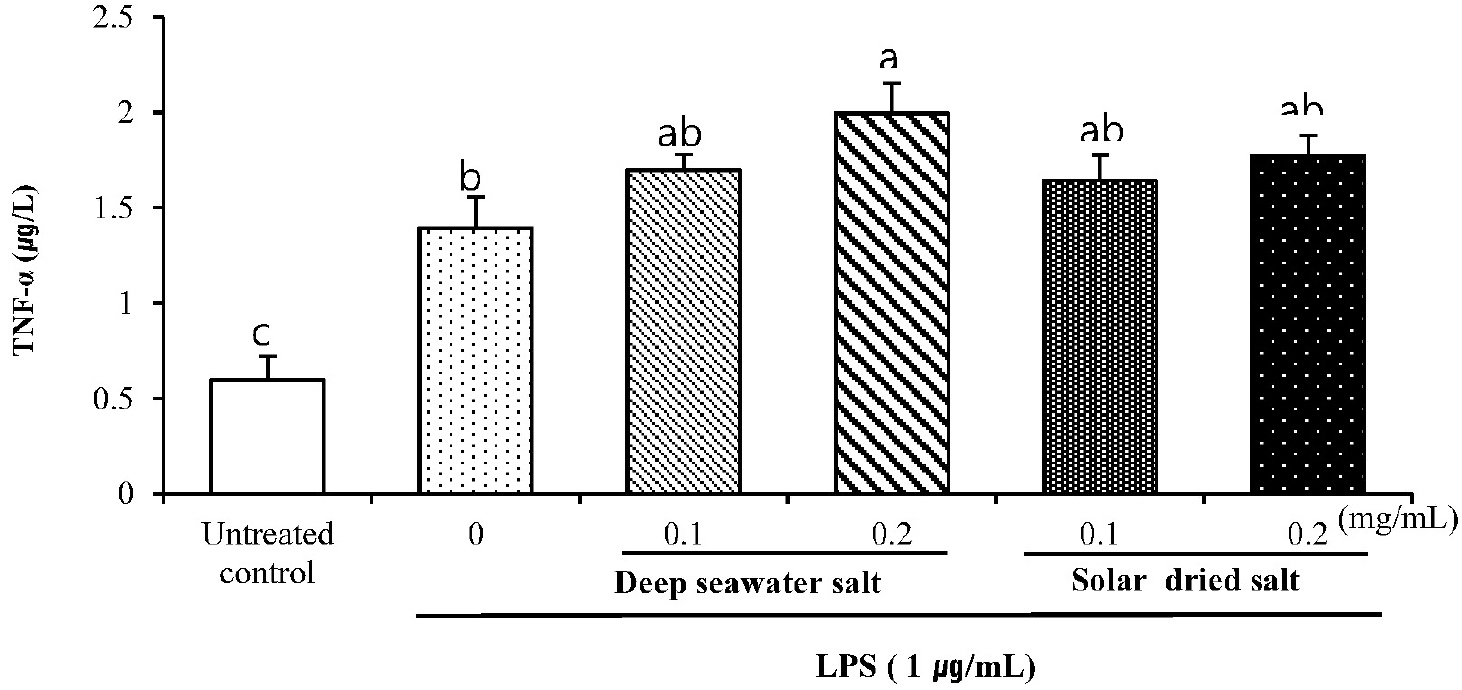
Fig. 4.
Effect of DS on secreted protein levels of TNF-α in LPS-induced RAW 264.7. Cells were pretreated with DS and SS (0.1 or 0.2 mg/mL) of for 18 h and exposed to LPS (1 µg/mL) for 24 h. Secreted protein level of TNF-α in the supernatant was measured by ELISA. Data are represented as means ± SD of three independent experiments.
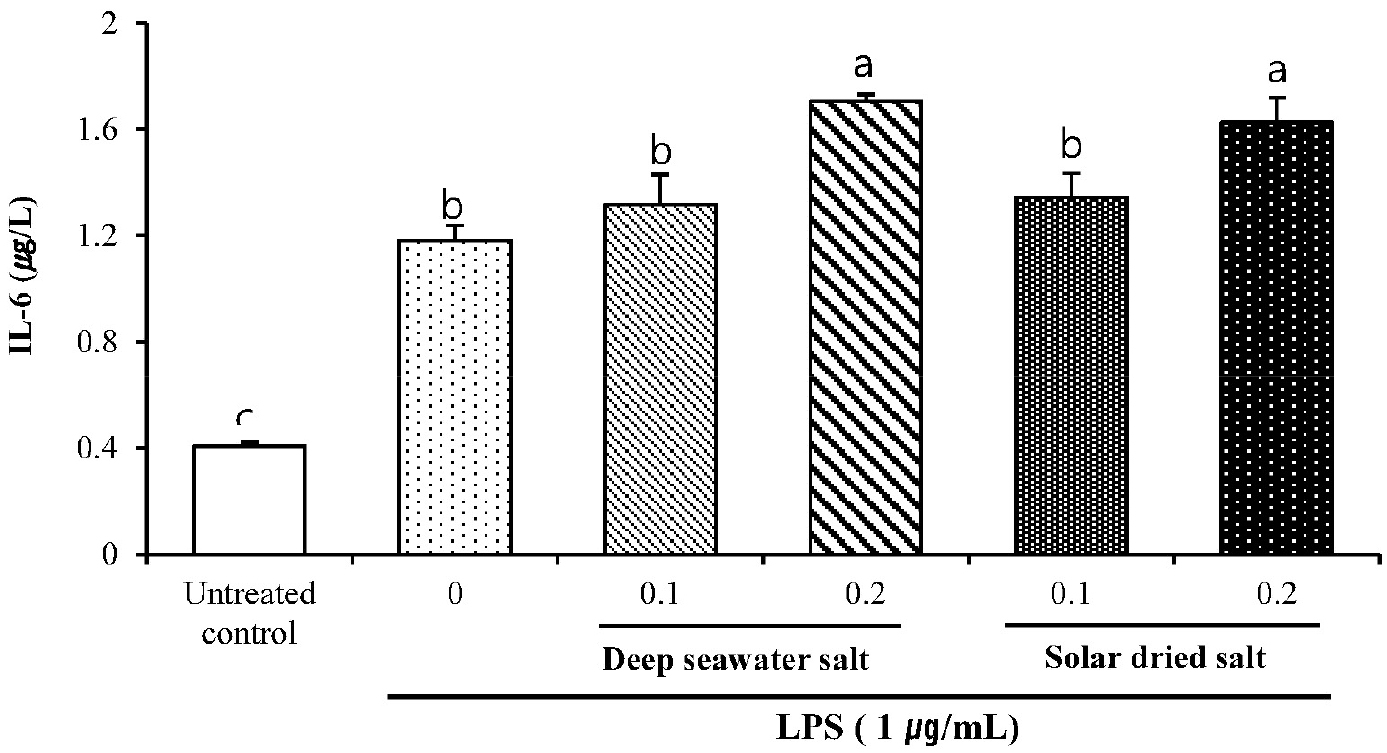
Fig. 5.
Effect of DS on secreted protein levels of IL-6 in LPS-induced RAW 264.7. Cells were pretreated with DS and SS (0.1 or 0.2 mg/mL) for 18 h and exposed to LPS (1 µg/mL) for 24 h. Secreted protein level of IL-6 in the supernatant was measured by ELISA. Data are represented as means ± SD of three independent experiments.
TNF-α has been recognized as an anti-tumor cytokine accompanied by serious toxicity and a cytokine stimulating phagocytosis on macrophage (Balkwill, 2009). IL-6 is an essential factor in the molecular control of antigen presenting cell development (Chomarat et al., 2000). Moreover, the induction of TNF-α, IL-6 and nitric oxide in unstimulated macrophage was considered as immune-stimulating effects of samples (Fang et al., 2012). From this, expressions of TNF-α and IL-6 cytokines are thought to be caused by promoting of immune system in macrophage cell, although overexpression of these pro-inflammatory cytokines can cause collateral damage to normal cells (Hamidzadeh et al., 2017).
As a result, it is suggested that significant increase of TNF-α and IL-6 nitric oxide in DS treated RAW 264.7 macrophage like cell line compared to those of other groups showed immune-stimulating activities of DS sample in vitro.
Discussion
In this study, it was tried to understand the physicochemical properties of deep seawater salt and then was focus on anti-inflammatory, anti-hypertensive and anti-osteoporosis effects.
Deep seawater salt contained more minerals, such as calcium, potassium, magnesium and manganese compared to solar dried salt. Deep seawater salt (DS) showed higher pH, reduction potential and contained more OH group compared to solar dried salt. DS showed anti-inflammatory effect, protein expressions of iNOS and COX-2. DS significantly increased in vivo NO production RAW 264.7. DS showed decreased the blood pressure in balb/c mice and revealed to prevent osteoporosis in SD rats compared to the solar dried salt.
Thus, it can be concluded by the followings from these studies
There are many mineral elements in DS, such as Na, Ca, Mg, Fe, Mn, P and K.
DS increased much better in vitro anti-inflammatory effect than solar dried salt by MTT assay.
The immune enhancing mechanisms of DS in the RAW 264.7 cells were inducing apoptosis by increasing apoptotic bodies and showing anti-inflammatory effects by regulating iNOS and COX-2 genes.
Summary and Future Implications
Thus, in this study, from the results of physicochemical property tests using various experimental methods, DS was a new salt substance different from the common salts. Because of more mineral compositions and new chemical construction, it increased immune enhancing effects DS can be used as functional foods from this study.